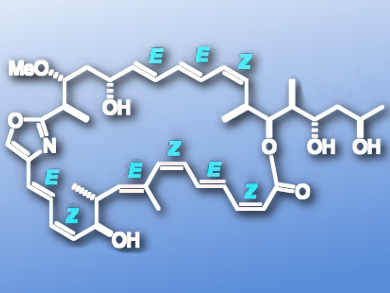
Chivosazole F (pictured) is a highly sensitive anticancer natural product. Ian Paterson’s group at the University of Cambridge, UK, has achieved a chemoselective and mild synthesis of chivosazole F. This 31-membered polyene macrolide contains a diene, triene, and tetraene with alternating double bond geometry, along with ten stereocentres. The team could execute a range of one-pot couplings, linking up to four highly functionalized fragments in a single operation to afford the full chivosazole backbone.
A series of transformations, including two stereoselective olefinations and an oxidation, then set the stage for the final Stille coupling. This closed the 31-membered macrocycle, completing the total synthesis in 20 steps and 2.5 % overall yield.
- An Expedient Total Synthesis of Chivosazole F: an Actin-Binding Antimitotic Macrolide from the Myxobacterium Sorangium Cellulosum,
Simon Williams, Jialu Jin, S. B. Jennifer Kan, Mungyuen Li, Lisa J. Gibson, Ian Paterson,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201610636

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

