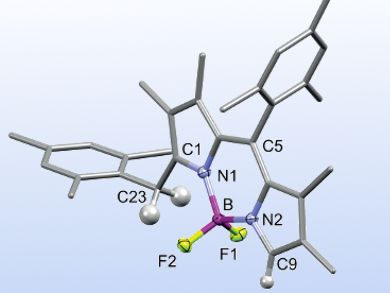
BODIPY (boron dipyrromethene) dyes are well-known for their small Stokes shifts and high quantum yields. Nonsymmetric derivatives, in particular, are important for the commercial success of BODIPYs in life science applications. They have been studied, e.g., as electroluminescence or near-infrared dyes. meso-Hydrogen-substituted derivatives are easily accessible; however, there is no general synthesis for nonsymmetric analogues yet.
Martin Bröring and colleagues, TU Braunschweig, Germany, have developed an efficient one-pot synthesis of nonsymmetric meso-aryl-BODIPYs. The team used 3,4-dialkylpyrroles with aromatic aldehydes in an acidic condensation, followed by BF2 complexation. Different electron-rich aldehydes can be employed to give the single nonsymmetric product, while electron-poor aldehydes give substantial amounts of the symmetric side product.
Additionally, the researchers investigated the reactivity profile of the BODIPY dyes. Chlorination of the free α-position can be achieved with different reagents. The chlorinated compounds can be used in nucleophilic substitution reactions with phenol, aniline, and thiophenols.
- One-Pot Preparation of Non-Symmetric meso-Aryl-BODIPYs: Functional Derivatives with Unusual Reactivity,
Clemens Cidarér, Martin Hoffmann, Julian Oelmann, Benedikt Wolfram, Martin Bröring,
Eur. J. Org. Chem. 2016.
DOI: 10.1002/ejoc.201601243