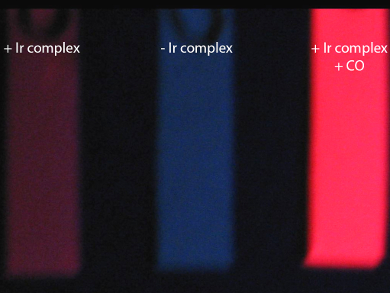
Exposure to carbon monoxide (CO) can lead to circulatory system failure, brain damage, and death. Early detection of this odorless and colorless gas can prevent accidental exposure. However, current detection technologies are often costly and have issues with long-term reliability.
Herbert Plenio, Technical University Darmstadt, Germany, and colleagues used iridium complexes such as [IrCl(cod)(bdp-NHC)] to detect CO. These complexes include a fluorescent molecule, bdp (1,3,5,7,8-pentamethyl-BODIPY), and a cyclooctadiene (cod) ligand. Reaction with CO leads to the substitution of the cyclooctadiene ligand, which then increases the fluorescence intensity of the bdp ligands. By exchanging the Cl– ion for different thiol-containing molecules, the researchers were able to tune the fluorescence response (up to an increase of 26 times after exposure to CO).
The team then tested the iridium complex using gaseous samples. Filter papers soaked with the complex showed a remarkable response to air with sub-lethal levels of CO (pictured right). The use of fluorescence could be a starting point for making simpler probes that detect harmful molecules in air.
- Fine-Tuning the Fluorescence Gain of FRET-Type (Bodipy)(Bodipy’)-NHC-Iridium Complexes for CO Detection with a Large Virtual Stokes Shift,
Oliver Halter, Israel Fernández, Herbert Plenio,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604757