Twisting Helices
Plants are capable of producing powerful movement that is initiated at the molecular level. This fast motion is often supported by helix-based architectures, for example in vetches or orchids that spread seeds by explosive opening of their pods. Researchers now demonstrate in the journal Angewandte Chemie that these biological strategies can be re-engineered by interfacing molecular switches with man-made materials.
Inspired by the evolutionary strategies that support the movement of plants, the team headed by Stephen P. Fletcher at the University of Oxford, UK, and Nathalie Katsonis at the University of Twente, Enschede, The Netherlands, joined together two strips of liquid-crystal elastomers into a pod-like casing. Light-triggered re-arrangement (isomerization) of a “molecular switch” incorporated in the material drives the twisting of the two valves in opposing directions, until the casing bursts open from strain.
Molecular Switch Drives Powerful Movement
“This is a man-made material in which the collective action of molecular switches produces powerful movement at the macroscale”, says Katsonis. “A slow and continuous movement will produce work over a large period of time, hence it offers low power density. If you confine work over a short period of time, you get more power.” With this plant-like molecular device the scientists demonstrate that it is possible to program molecular materials to perform complex tasks. Says Katsonis: “Ultimately and in the long term we hope that life-like materials could promote a transition towards adaptive, energy efficient, and sustainable materials.”
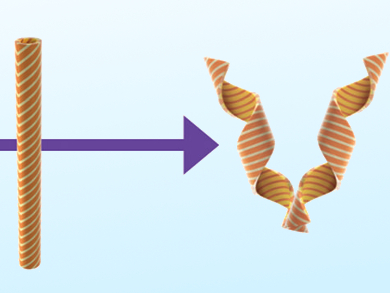
The design of the pods involves cross-linked liquid crystals, because they are capable of directional (anisotropic) shape transformation. Using a two-step photo-polymerization process, the researchers created a thin film of liquid-crystal elastomer consisting of bars with alternating low and high ordered liquid-crystalline states. Then, strips are cut out of this film. When irradiated with UV light, the molecular switches integrated in the elastomer isomerize from their straight to their bent form. This causes the highly ordered bars to shrink along their long axis—much more than the disordered bars do, resulting in a difference in length between the bars.
The angle at which the film is cut determines what happens macroscopically: strips cut at 0° or 90° to the long axis bend without a twist when irradiated. Cuts at 45° or 135° result in helical strips that wind under illumination—with opposite directions of twist. The researchers brought two mirror-image strips together. Under irradiation, the pod bends along its long axis, then forms a tube along its transverse axis, in which the mirror-image helices work against each other and strain accumulates, until the pod pops open from stress. “The accumulation of strain in tubular architectures is an elegant engineering strategy that is common in nature, and here it allows to amplify the action of a few dynamic molecules, and transforms it into powerful movement.”
- High-Power Actuation from Molecular Photoswitches in Enantiomerically Paired Soft Springs,
Sarah J. Aßhoff, Federico Lancia, Supitchaya Iamsaard, Benjamin Matt, Tibor Kudernac, Stephen P. Fletcher, Nathalie Katsonis,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201611325