Lanthanide fluorides and oxyfluorides are ideal as fluorescent hosts owing to their low vibrational energies and wide band gap (>10 eV). Systematic research on the morphology-controlled synthesis of nano/micromaterials has been driven by their intrinsic morphology/size-dependent properties. Among the lanthanide derivatives with diversiform morphologies, porous spheres are more desirable for biomedical and energy-related applications owing to their high drug-loading space and high specific surface.
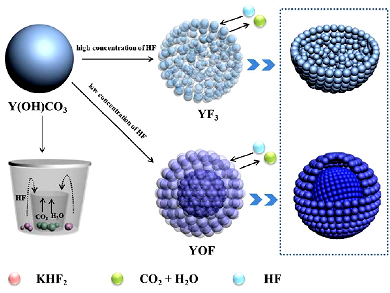
Hongpeng You, Chinese Academy of Sciences, Changchun, China, and colleagues have synthesized monodisperse YF3 and YOF porous sub-microspheres by a sacrificing template method using amorphous Y(OH)CO3·xH2O as precursors as well as the template. The luminescence properties of the as-synthesized YF3:Ln3+ (Ln = Eu, Tb, Ce, Ce/Tb, Yb/Er, Yb/Ho, and Yb/Tm) showed strong multicolor emissions including down/upconversion and energy-transfer processes. YF3 and YOF inherited the spherical shapes and monodispersity of their precursors, while the final products had different pore structures by tuning the amounts of the fluorine source.
A series of contrast experiments on the formation processes of these pore structures showed that the difference in the initial concentration of HF gas was responsible for the YF3 homogeneous porous structure and YOF yolk-shell like structure. Moreover, YOX (X = Cl and Br) could be obtained when the calcination atmosphere was a halogen. This strategy was also extended to other lanthanide fluorides (REF3, RE = Gd, Lu), which may open a facile way to fabricate other porous nanostructures.
- Facile Synthesis of Lanthanides (Ce, Eu, Tb, Ce/Tb, Yb/Er, Yb/Ho, and Yb/Tm) Doped LnF3 and LnOF Porous Sub-microspheres with Multicolor Emissions,
Shuang Zhao, Baiqi Shao, Yang Feng, Senwen Yuan, Jiansheng Huo, Wei Lv, Kai Liu, Hongpeng You,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201701142