The quest to systemize organic reactivity dates back to the 1920s when the terms nucleophile and electrophile were first introduced. Since then, many studies have focused on establishing universal reactivity scales in order to facilitate synthesis planning.
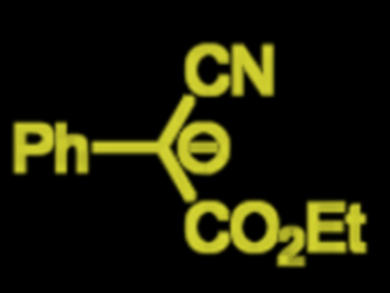
Herbert Mayr, University of Munich, Germany, and colleagues have established a nucleophilicity scale that currently classifies 1066 nucleophiles. Their most recent study focuses on the nucleophilicity of bisacceptor-substituted benzyl anions (example pictured). Kinetics of reactions between the benzyl anions and different reference electrophiles were studied. They were determined to be second-order; first order with respect to the electrophile and to the carbanions. Phenyl groups usually stabilize charges by the mesomeric effect, but the team found that the stabilizing effect of two acceptor groups at a carbanionic center is hardly enhanced by an additional phenyl group.
The team used a linear correlation of the type lg k2(20 °C) = sN(E + N) , which characterizes nucleophiles by two solvent-dependent parameters sN and N and electrophiles by one parameter E (k2 = rate constant). This allows the team to integrate the benzyl carbanions in the previously established nucleophilicity scale, which enables a direct comparison of different compound classes.
- Nucleophilic Reactivities of Bis-Acceptor-Substituted Benzyl Anions,
Ángel Puente, Armin R. Ofial, Herbert Mayr,
Eur. J. Org. Chem. 2017, 2017, 1196–1202.
DOI: 10.1002/ejoc.201601513