Hydroxide Surrogate
The rational design of a new reagent, a hydroxide surrogate, could make the conversion of aryl halides to phenols under mild conditions far less the formidable challenge they currently are, according to work published in Angewandte Chemie.
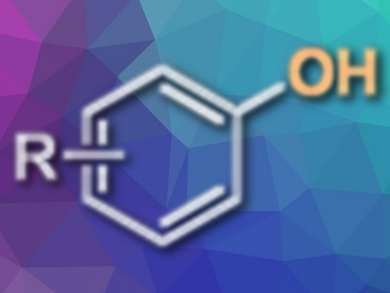
Phenols and their derivatives are widespread throughout the chemical industry from fine chemicals to pharmaceuticals, agrochemicals, and the products of materials science. They are also widely used intermediates. Despite their great utility, however, there are very few methods that can cope with forming a new bond between an aryl group and a hydroxyl without harsh conditions that wreak havoc on the complex and sensitive functionality that is commonly present in a target compound.
Softening the Harsh Conditions
Patrick Fier and Kevin Maloney of the Department of Process Research and Development at Merck & Co., Inc. in Rahway, New Jersey, USA, may have remedied this situation with their development of a palladium-catalyzed method to prepare phenols. In their approach, benzaldehyde oxime acts as a surrogate molecule for the normally harsh hydroxide. The team explains that the direct hydroxylation of an aryl halide is perhaps the most attractive way in which to make a novel phenol because haloarenes are so common.
Copper-based catalysts are usually used for this, but these catalysts require high temperature and a strong base to proceed efficiently and effectively. Moreover, they are generally limited to aryl iodides and bromides, the team points out. More expensive palladium-based catalysts might be used instead and have a wider repertoire of products, but they, too, need cesium hydroxide, potassium hydroxide, or an organic superbase to work well. This precludes the use of base-sensitive substrates.
Fier and Maloney point out that even with a palladium catalyst, hydroxide coupling cannot occur when functional groups that can undergo N– or O-arylation are present, which excludes amides, amines, and alcohols from plausible reaction schemes.
Basic Solution
The researchers have rationally designed a palladium-based catalytic method that circumvents all of these problems by removing the need for a strong base and side-stepping the catalytic intermediate containing an Ar-Pd-OH (aryl-palladium-hydroxide) grouping.
There were five criteria that a surrogate for hydroxide would have to meet in their scheme, the team says. First, it must work under mildly basic conditions. Secondly, the formation of the intermediate should liberate the phenol product with only innocuous byproducts. Thirdly, the reaction would have to be tolerant of electrophilic and base-sensitive functional groups in the system. The fourth consideration would be that it should still be able to react even in the presence of the phenol products and nucleophilic sites in the substrates. Finally, the hydroxide surrogate would have to be readily available “off-the-shelf”, stable, and inexpensive to make the reaction commercially viable.
Benzaldoxime as a Surrogate
With these criteria in mind, the team identified benzaldoxime as a likely candidate for a mild hydroxide surrogate. This compound would react with aryl halides to form an O-aryl oxime intermediate. The release of the phenol product would be accompanied by the formation of nothing more noxious to the reaction than benzonitrile. Benzaldoxime has an appropriate acidity, e.g., in dimethyl sulfoxide – close to that of phenol. Thus, it functions in the reaction under mildly basic conditions but is itself far less basic than hydroxide.
High-throughput experiments allowed the researchers to home in on suitable solvents, bases, temperatures, and stoichiometries. The team carried out 96 experiments to test their most promising leads – cesium carbonate and potassium phosphate in dimethyl formamide or 2-methyl-tetrahydrofuran and explored 24 palladium catalyst candidates. These tests identified conditions that allow the synthesis of complex phenols with sensitive functional groups.
“We anticipate that this methodology will be rapidly adopted due to the mild conditions and the ability to perform the reaction on functionally dense complex molecules,” the team concludes.
- Synthesis of Complex Phenols Enabled by a Rationally Designed Hydroxide Surrogate,
Patrick S. Fier, Kevin M. Maloney,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201700244