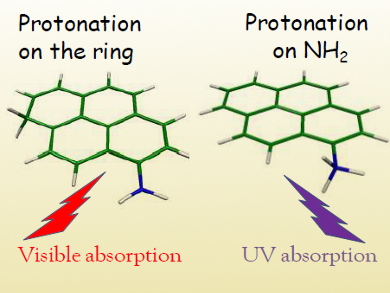
The protonation of aromatic systems bearing amino groups in solution is generally expected to occur preferentially on the amine, whereas protonation of a carbon atom in the aromatic ring is less likely.
Christophe Jouvet, Aix-Marseille University, France, and colleagues have studied the photofragmentation of protonated 1-aminopyrene in a cold ion trap and observed tautomers protonated on both the amino group and the carbon ring (pictured). The amino tautomer presented more intense bands, indicating that it was the major species.
Gas phase calculations suggest that the most stable protonation sites for this molecule are the aromatic carbon atoms. This study also showed that the molecule’s πσ* electronic state can explain the observed photoreactivity of aromatics in acidic solution. Direct proton transfer to form H3O+ would require 10 eV and is thus highly unfavorable. The πσ* state lying at 4.2 eV, on the other hand, could lead to hydrogen transfer to a H3O radical, followed by electron transfer to form the reaction product H3O+.
- Electronic spectroscopy of protonated 1-aminopyrene in a cold ion trap,
Christophe Jouvet, Jennifer Anna Noble, Claude Dedonder-Lardeux, Joëlle Mascetti,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700327


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
