Autonomous Fuel-Cell-Based Power Source
Miniaturized devices such as microsensors often require an independent, equally miniaturized power supply. Searching for suitable systems, Japanese scientists have developed a fully integrated microfluidic device that produces hydrogen fuel and converts it into electrical energy based on photocatalysis. As they report in the journal Angewandte Chemie, it works fully autonomously and delivers enough hydrogen energy to power a microsensor for daily data transmission.
Downsizing has its challenges, especially when miniaturized autonomous systems like lab-on-a-chip applications or microsensors are demanded. These systems often need their own power supply, but external batteries are clumsy and difficult to integrate. Microfluidic systems offer such integration.
Takehiko Kitamori and Yuriy Pihosh, University of Tokyo, Japan, and colleagues have designed a photocatalytic microgenerator of hydrogen fuel, combined with a micro fuel cell, all set up on a microfluidic chip. This microfluidic power generator is based on sunlight and can provide a continuous power supply to other miniaturized devices at room temperature and at atmospheric pressure, it is claimed.
Fuel Cell and Fuel Generator Modules
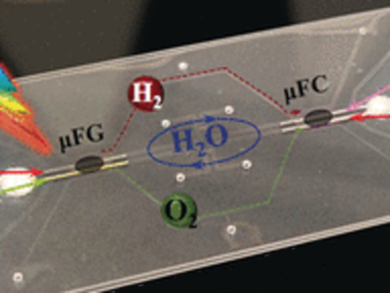
The scientists describe their microfluidics power device as a modular system set on a glass platform with the two modules, the photocatalytic micro fuel generator and the micro fuel cell, being connected by a set of micro- and nanochannels. Both microfluidic modules contain a set of “extended nanochannels” for proton exchange—the researchers argue that these ENCs provide an excellent proton conductance and allow much faster proton travel than the conventional Nafion proton exchange membranes.
The photoanode, namely, the photocatalyst for water splitting, is also innovative: it consists of especially designed metal-oxide nanorods that photocatalyze the production of hydrogen with “record efficiency”, as the team has demonstrated. Both gases produced by water splitting, oxygen and hydrogen, are then separately transported through the microchannels to the micro fuel cell, where oxygen, electrons, and protons electrochemically combine to water, providing the energy.
Power for Microsensors or Lab-on-a-Chip Applications
As the water is circulated back to the first module, this micro power supply is self-sustaining and only dependent on sunlight. The scientists tested the device and found a steady hydrogen production per day, which is “equivalent to 35 millijoules of stored energy that would be enough to power a microsensor and transmit time data during 24 hours,” they said. Still, they have to integrate a set of microtanks for gas storage to avoid overpressure of the gases, but according to the authors, this issue can be addressed quickly.
Applications suggested are autonomous microsensors and lab-on-a-chip technologies, the latter of which can downsize entire laboratory processes, thereby saving valuable material and energy costs.
- From Extended Nanofluidics to an Autonomous Solar-Light-Driven Micro Fuel-Cell Device,
Yuriy Pihosh, Jin Uemura, Ivan Turkevych, Kazuma Mawatari, Yutaka Kazoe, Adelina Smirnova, Takehiko Kitamori,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703227