Free radicals are highly reactive species that can directly damage DNA, proteins, and lipids and induce cancer cell death. Among the existing free-radical therapeutic modalities, photodynamic therapy (PDT) is the most predominant strategy. Energy from light can be transferred by photosensitizers (PSs) to tumor oxygen to generate toxic reactive oxygen species (ROS) for cancer therapy. However, the production of ROS is highly oxygen-dependent. By this, the therapeutic efficacy of photodynamic therapy can be greatly hindered by tumor hypoxia. Tumor hypoxia leads to resistance to cancer therapy and might increase tumor metastases. To solve this problem, oxygen-independent strategies that can generate free radicals are highly desirable in cancer therapy.
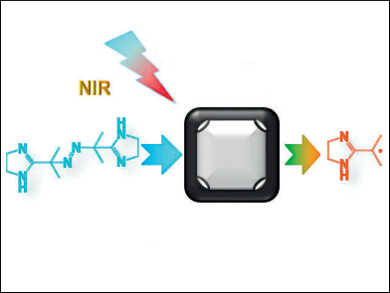
Xian-Zheng Zhang, Wuhan University, China, and colleagues have designed a method for generating free radicals that is induced by near-infrared light. A thermally decomposable initiator, 2,2-azobis[2-(2-imidazolin-2-yl)-propane] dihydrochloride, was used as a free-radical source, while gold nanocages were used as both the initiator carrier and heat source. The initiator was loaded into the hollow cavities of the nanocages. Upon near-infrared light irradiation, the photothermal effect from the nanocages increased the surrounding temperature, and subsequently accelerated the decomposition of the initiator to generate alkyl radicals. The generated radicals were then released in tumor cells.
This concept worked well both in vitro and in vivo. The free radicals effectively caused oxidative stress and DNA damage, leading to cancer cell death under different oxygen tensions (normoxic and hypoxic conditions). According to the researchers, this work could open up a new field for the use of free radicals in cancer therapy.
- Initiator-Loaded Gold Nanocages as a Light-Induced Free-Radical Generator for Cancer Therapy,
Xiao-Qiang Wang, Fan Gao, Xian-Zheng Zhang,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703159