Removing Oil by Absorption
Spilled crude oil has repeatedly polluted and even destroyed marine ecosystems. An effective measure would be to remove spilled oil slicks by absorption into a separable solid phase. As Indian scientists report in the journal Angewandte Chemie, congelation of the oil to a rigid gel within impregnated cellulose and scooping the particles out is possible.
Marine oil spills are disasters that cannot be completely avoided as long as we drill for oil or transport it across the ocean. As oil slicks pose huge environmental and economic threats, people try to recover the floating spilled oil from the water surface before it reaches the shores or is emulsified by a turbulent sea. But this is difficult. Simply skimming or booming often fails as the oil film quickly spreads out in large areas.
Combining Absorption and Gelation
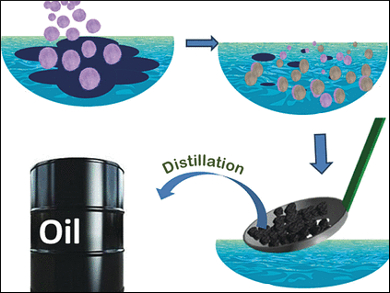
Kana M. Suresan and Annamalai Prathap from the Indian Institute of Science, Education and Research (IISER) Thiruvananthapuram in Kerala, India, have developed and tested an intriguingly simple strategy. Combining absorption and gelation processes, they tightly bound the oil to a porous matrix and then simply scooped the solid particles out of the water. Even full with the oil, the granules did not sink but remained at the surface.
The scientists chose cellulose as an environmentally friendly, cheap, and porous carrier matrix and impregnated it with a so-called oleogelator, which is a cheap mannitol-based organic compound. This simple impregnation step proved to be key in converting the cellulose to an effective oil-absorbing and recycling system.
Practical and Eco-Friendly Recovery
The first reason for that is the gelation ability of the gelator. “Phase-selective organogelators are amphiphiles which can congeal oils selectively from a biphasic mixture of oil and water,” the scientists explain. Gelation occurs because the gelator molecules get dissolved in the oily phase, and then they form a three-dimensional fiber network through hydrogen bonding. The oil becomes trapped in this fibrillar network to form a rigid gel. Thus, gelation turns the liquid oil phase into a solid one, which can be simply scooped out.
The other advantage of impregnation is that the gelator renders the cellulose matrix hydrophobic. It did not suck in water as naked cellulose does. But it “absorbed all the oil, and the rigid globules containing the congealed oil could be scooped out after two hours, leaving the clean water,” the researchers reported. And even recycling was possible: The scientists demonstrated that squeezing or distillation of the congealed granules can yield the spilled oil. This simple, cheap, and environmentally benign system will add interesting aspects for further field research.
- Organogelator-Cellulose Composite for Practical and Eco-Friendly Marine Oil-Spill Recovery,
Annamalai Prathap, Kana M. Sureshan,
Angew: Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201704699