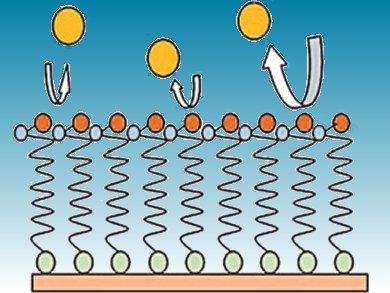
Gold-based biosensors require passivated surfaces to reduce the electrochemical signal from gold and avoid fouling of the surface. To better understand the passivation process, Osvaldo Oliveira and colleagues, Universidade de São Paulo, Brazil, have monitored the film growth and organization of 11-mercaptoundecanoic acid (MUA) and cysteamine monolayers on a gold surface.
They found that cysteamine was an ineffective passivating agent, but MUA completely blocked the gold substrate after 48 h of immersion in a MUA and ethanol solution. The MUA underwent molecular reorganization to form a monolayer with alkyl chains perpendicular to the gold surface which lead to its passivating activity.
Such precise monitoring of the buildup of films is needed to design stable self-assembled monolayers with useful properties, including nonfouling surfaces for biosensors and improved coatings for photovoltaic devices.
- Probing the Functionalization of Gold Surfaces and Protein Adsorption by PM-IRRAS
R. Marques de Oliveira, J. Ferreira, M. J. L. Santos, R. M. Faria, O. N. Oliveira,
ChemPhysChem 2011.
DOI: 10.1002/cphc.201100080