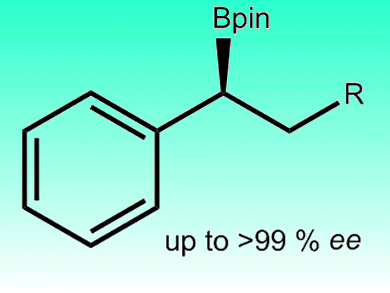
Hydroboration of unsaturated carbon–carbon bonds is a valuable synthetic method for the preparation of organoborane intermediates. Most hydroborations are performed with rhodium, however, a copper(I) complex has been found to be an effective catalyst for asymmetric hydroboration with pinacolborane, by Jin Yong Lee, Jaesook Yun, and co-workers, Sungkyunkwan University, Korea. The catalyst worked with β-substituted styrene derivatives and afforded benzylic pinacolboronate esters with excellent regio- and enantioselectivities (up to >99 %).

Experimental and theoretical studies were conducted to probe the reaction mechanism; cis addition of Cu–H and stereoretentive transmetalation via σ-bond metathesis was proposed based on a deuterium labeling study. Vinylarene substrates with a substituent at the β-position are challenging to hydroborate and this Cu catalyst provides an excellent alternative to more expensive asymmetric rhodium catalysts.
- An Efficient Copper(I)-Catalyst System for the Asymmetric Hydroboration of β-Substituted Vinylarenes with Pinacolborane
D. Noh, S. K. Yoon, J. Won, J. Y. Lee, J. Yun,
Chem. Asian J. 2011.
DOI: 10.1002/asia.201100146