In the drive for clean energy, hydrogen is seen as a promising candidate for energy storage or as a fuel. It can be produced by splitting water, which requires the use of a photocatalyst or electrocatalyst. The best catalyst for the hydrogen evolution reaction (HER) is metallic platinum. However, its rarity and high price cause a need for cheaper, more abundant catalyst materials.
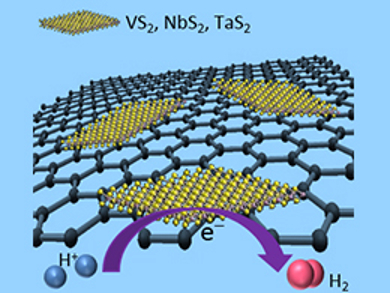
Martin Pumera, Nanyang Technological University, Singapore, and colleagues have investigated composite materials as alternative HER catalysts. The team combined graphene with sulfides of Group 5 metals (VS2, NbS2, and TaS2). Mixed graphene oxide and metal salts were heated under hydrogen sulfide gas to produce the composites (pictured).
The catalysts’ properties were measured under standard conditions for comparison with other materials. All composites show improved HER performance compared with graphene oxide and with the bulk-produced sulfides, demonstrating the enhancement effects of composite materials. The VS2 composite has the best performance, with an overpotential of 0.77 V and a Tafel slope of 106 mV dec–1. Although these figures are nowhere near as good as for platinum (0.05 V, 38 mV dec–1), this study shows the potential of Group 5 sulfides as hybrid electrocatalysts for HER.
- Graphene/Group 5 Transition Metal Dichalcogenide Composites for Electrochemical Applications,
Yong Wang, Zdeněk Sofer, Jan Luxa, Xinyi Chia, Martin Pumera,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201701843