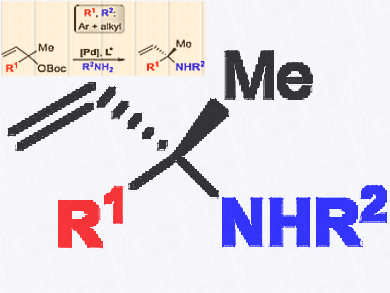
Chiral α,α-disubstituted allylic N-alkyl amines are important building blocks in organic synthesis and medicinal chemistry. However, to date, there is no general synthesis approach.
Arjan W. Kleij, Institute of Chemical Research of Catalonia (ICIQ), the Barcelona Institute of Science and Technology, Tarragona, Spain, and colleagues have reported the first asymmetric synthesis of α,α,α-disubstituted N-alkyl allyl amine scaffolds. The synthesis uses palladium catalyzed allylic substitution, is user-friendly, and can be done under mild and additive-free conditions.
The scope of the product is the widest reported so far for these allylic amine scaffolds. High asymmetric induction with the enantiomeric ratio (er) up to 98.5:1.5 are found. The use of less reactive anilines is also feasible providing enantioenriched α,α,α-disubstituted N-aryl allylic amines.
According to the researchers, their synthesis method is the most effective method reported to date, although some substrate combinations deliver moderately high asymmetric induction levels and some limitations are pertinent to the allylic precursors. In addition, their work highlights the key role of steric modulation in the allylic substrate to prepare chiral allylic amines via palladium catalysis.
- Asymmetric Synthesis of alfa,alfa-Disubstituted Allylic Amines via Pd-Catalyzed Allylic Substitution,
Arjan Willem Kleij, Wusheng Guo, Aijie Cai, Jaining Xie,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201705825