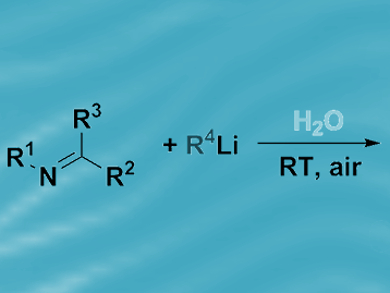
For more than a hundred years, organic chemists have handled organolithium and Grignard reagents under an inert atmosphere, using aprotic, dry organic solvents and low temperatures (–78 °C). Such rigorous experimental techniques are deemed necessary because of the sensitivity of the highly polar metal–carbon bonds contained within such compounds.
Vito Capriati, Università di Bari, Italy, and colleagues have discovered that water can be used as a reaction medium for fast and chemoselective nucleophilic additions of Grignard and organolithium reagents to imines and nitriles at room temperature and in air. Secondary amines and tertiary carbinamines were isolated from vigorously stirred reaction mixtures in high yields.
The researchers ascribe the successful outcome of these so-called “on-water” catalytic transformations to water’s strong hydrogen-bonding ability, which is thought to enable proton transfer across the organic–water interface. Reagent deactivation by protonolysis was more prevalent in methanol, in which the intermolecular hydrogen bonding is weaker. Polar organometallic reagents are notorious for their sensitivity; however, these findings suggest there may be synthetic opportunities in water.
- Unprecedented Nucleophilic Additions of Highly Polar Organometallic Compounds to Imines and Nitriles Using Water as a Non-Innocent Reaction Medium,
Giuseppe Dilauro, Marzia Dell’Aera, Paola Vitale, Vito Capriati, Filippo Maria Perna,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201705412