Many properties of crystalline materials, such as their catalytic and optical characteristics or the processability of particles, are linked to their morphologies. Crystal shape can be controlled with soluble additives, although the way this works is not yet fully understood because crystallization processes can be very rapid and challenging to monitor.
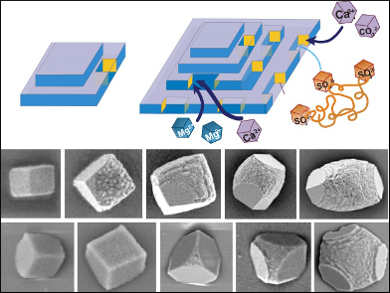
Yi‐Yeoun Kim, Fiona C. Meldrum, University of Leeds, UK, and colleagues have studied the influence of the additives Mg2+ and poly(styrene sulfonate) (PSS) on crystal growth from the earliest stages of crystallization. A microfluidic device was used to precipitate crystals of calcium carbonate in nanoliter volumes, where growth occurs more slowly. The team discovered that, as a calcite crystal grows, the density of favorable, high-energy sites for additive binding increases.
However, shape changes (examples pictured) only become evident when the concentration of such sites is great enough, typically once the crystals reach sizes of 0.1–0.5 μm for Mg2+ and 1–2 μm for PSS. These results suggest that additives affect crystal morphology at a much later stage than previously thought. Such findings could be useful for optimizing industrial crystallization processes.
- The Effect of Additives on the Early Stages of Growth of Calcite Single Crystals,
Yi-Yeoun Kim, Colin L. Freeman, Xiuqing Gong, Mark A. Levenstein, Yunwei Wang, Alexander Kulak, Clara Anduix-Canto, Phillip A. Lee, Shunbo Li, Li Chen, Hugo K. Christenson, Fiona C. Meldrum,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201706800




Congratulations