Our car’s exhaust is causing damage to each of us. The pictures below explain the chemical composition of a car’s exhaust gas.
1 Chemical Composition of a Car’s Exhaust Gas
.jpg)
CO binds to hemoglobin stoping the oxygen transport in the blood. Poisoning is lethal within a short time.
NO oxidizes rapidly in the air to NO2, a poisonous, cough-inducing gas. Above 21 °C, N2O4, a corrosive and strongly oxidizing gas, is produced.
.jpg)
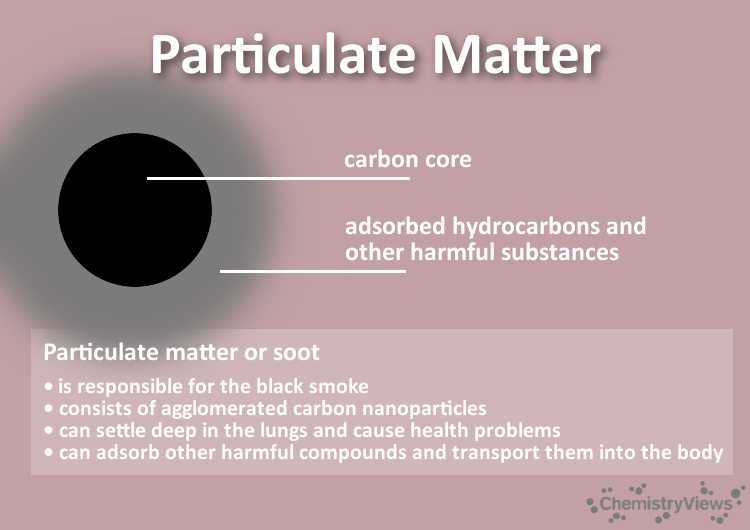
Once inhaled, these small particles remain in the body forever. They can pass through the membranes of the human pulmonary vesicles and thus enter the blood circulation. On their surface, they can introduce solid and liquid substances from the combustion process into the bloodstream.
See also Particulate Matter (Clever Picture), ChemViews Magazine 2018; https://doi.org/10.1002/chemv.201800012.
2 Types of Combustion
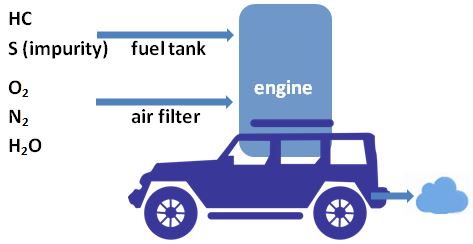
To understand where the above-shown exhaust gas comes from, we look at what happens when fuel is burned with air in the engine:
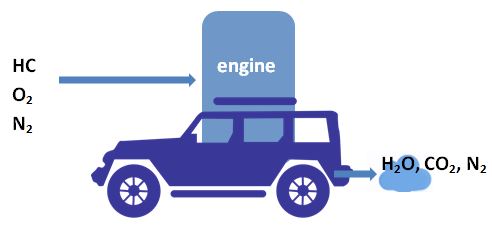
2.1 Perfect Combustion
Hydrocarbons (HC) are oxidized into water and carbon dioxide. Nitrogen passes unaffected.
2.2 Incomplete Combustion
Combustion in the engine is always incomplete, especially when starting the car or during acceleration.
Quenching: When the combustion flame front comes in contact with the relatively cool walls of the combustion chamber the flame is extinguished before all the fuel is burned. Hydrocarbons are emitted.
Rich air/fuel mixture: When there is not enough oxygen present in the air/fuel mixture during combustion the carbon from HC is only partially oxidized and CO is emitted.
At high combustion temperatures, NOx is formed.
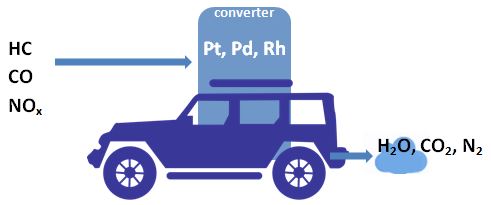
Exhaust Gas Treatment
The emissions occur although today’s exhaust gas is treated by a catalytic converter.
The exhaust gases of petrol-fuelled cars is treated with a three-way catalytic converter. Unburnt hydrocarbons, CO, and NOx are converted to CO2, H2O, and N2 with Pt, Pd, and Rh catalysts. The exhaust gas of diesel-powered cars is treated with a two-way catalytic converter. The oxygen in the exhaust gas of diesel vehicles makes the conversion of NOx inefficient.
If you want to drive cleaner, you can either ask for Euro 6c when buying a new car, buy a natural gas vehicle, … or use a bike.
Sources
- Uptake and intracellular accumulation of diamond nanoparticles – a metabolic and cytotoxic study,
Antonín Brož, Lucie Bačáková, Pavla Štenclová, Alexander Kromka, Štěpán Potocký,
Beilstein J. Nanotechnol. 2017, 8, 1649–1657.
DOI: 10.3762/bjnano.8.165
- Combustion Chemistry, Toyota Motor Sales, U.S.A., Inc., Plano, TX, USA.
- Exhaust Emissions, Volkswagen AG, Wolfsburg, Germany 1998.
Also of Interest
- Particulate Matter,
ChemViews Mag. 2018.
https://doi.org/10.1002/chemv.201800012