Metal nanoparticles are useful for a number of applications, most prominently in the field of catalysis. Colloidal dispersions of metal clusters are often synthesized from a homogeneous metal salt in a dispersion medium; however, they must be stabilized to prevent aggregation. One method of stabilizing metal nanoparticles is by using metal complexes as the precursor, which produces nanoparticles with ligands coordinated to the surface. This method can also produce desirable characteristics in the nanoparticles, such as solubility in certain media.
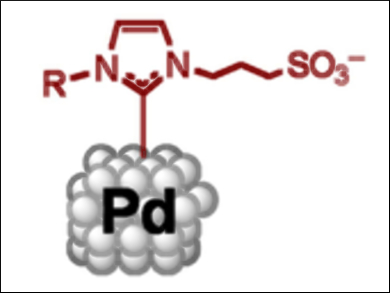
Ernesto de Jesús, Universidad de Alcalá, Spain, and Bruno Chaudret, Université de Toulouse, France, and colleagues have synthesized water-soluble palladium nanoparticles. Using N-heterocyclic carbene (NHC) ligands featuring sulfonate groups, stable palladium nanoparticles that are soluble in water (pictured) were prepared. This could be achieved through three methods: thermal decomposition of the precursor, reduction under a CO atmosphere, or reduction under an H2 atmosphere.
The resulting nanoparticles could be used in the catalytic hydrogenation of styrene. By simply using the dispersion as a reaction medium, 97 % conversion could be achieved after three hours. The nanoparticles could also be recycled for ten catalytic cycles. This stability is an important result and may be useful for the production of recyclable catalysts in sustainable reaction media.
- Synthesis of Water-Soluble Palladium Nanoparticles Stabilized by Sulfonated N-Heterocyclic Carbenes,
Juan M. Asensio, Simon Tricard, Yannick Coppel, Román Andrés, Bruno Chaudret, Ernesto de Jesús,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201702204