Carbon dioxide and C2–C3 alkanes are well-known greenhouse gases generated by human activities. Therefore, it is highly desirable to develop chemical processes that can jointly use CO2 and C2–C3 alkanes in chemical synthesis.
Vadim V. Guliants and colleagues, University of Cincinnati, OH, USA, have synthesized Na-promoted vanadia catalysts supported on silica and investigated their behavior in propane oxidative dehydrogenation (ODH) reactions using O2 and CO2.
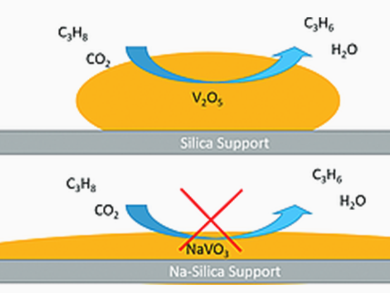
Propane ODH by O2 and CO2 was performed over Na-free and Na-promoted supported VOx/SiO2 catalysts. Typically, O2 is used in ODH. CO2 has a lower catalytic activity, which means that more reactive olefins are less likely to undergo undesirable combustion in its presence. The researchers found, that promotion with Na+ significantly improved the dispersion of surface VOx species on silica. However, it resulted in the formation of surface Na metavanadate species or another reduced V3+/V4+ phase, which lacks catalytic activity in propane ODH in the presence of CO2.
Raman spectroscopic studies revealed a trend that with increasing vanadium content on silica, 2D vanadia structures are formed at first. Then, the formation of 3D vanadia crystalline structure is favorable. Thus, the maximum amount of 2D vanadia structure that shows high activity for CO2 ODH of propane was obtained in a monolayer-coverage range. Considerable carbon deposition was observed in used catalysts after propane ODH by CO2, the extent of which correlated with their catalytic activity.
- Carbon Dioxide as Feedstock in Selective Oxidation of Propane,
Jungshik Kang, Andrew D. Czaja, Vadim V. Guliants,
Eur. J. Inorg. Chem. 2017.
https://doi.org/10.1002/ejic.201701049