In public and private buildings as well as in transport vehicles, people are confronted with a broad spectrum of foreign substances, via both the air and dust residues. In this part, we take a look at man as a source of substances and at life on Mars.
7. Transdermal Substance Uptake: “Dr. No” as a Scientific Role Model?
The previous chapter naturally raises the question as to whether substances in the air can be absorbed directly through the skin. In this regard, an expedient experimental design involving the viewing of films intended for entertainment purposes can actually be quite useful. Ingrained James Bond fans are certainly not the only ones who are familiar with the super-villain “Dr. No”. As a typical “mad scientist”, he carries out mischief with radioactive material on a remote Caribbean island. For personal protection, Dr. No wears a cover made out of plastic in his laboratory. Though there is every reason to doubt the shielding power of plastic relative to radioactive emission, the general principle is marvelously well suited for exposure experiments (see Fig. 14).
 |
|
Figure 14. “Dr. No” as a scientific role model.
|
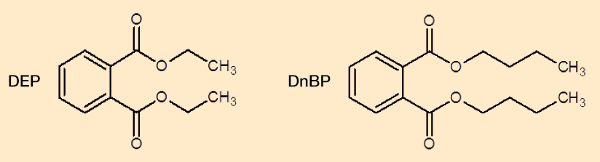
Diethyl phthalate (DEP) and di-n-butyl phthalate (DnBP) (see Fig. 15) fall in the category of semivolatile organic compounds (SVOC), which are assumed capable of being absorbed into the human body to a significant extent directly from the air, via the skin. The goal of an international investigation was to determine experimentally the true extent of transdermal absorption of DEP and DnBP directly from the gas phase by measuring the level of metabolites in urine. This was accomplished by repeated controlled exposure of test persons in a large test chamber, measuring uptake both including and excluding that from inhalation.
 |
|
Figure 15. Structures of diethyl phthalate (DEP) and di-n-butyl phthalate (DnBP). |
Experiment 1
The subjects, six healthy males between 27 and 66 years of age, clad only in shorts, spent six hours on two separate days in the test chamber. Aluminum plates coated with a phthalate-containing paint served to ensure continuous dosing of DEP and DnBP into the chamber air. Between 12 hours before the start of each experiment and 48 hours after the end of the process, each subject maintained a strict diet, and avoided articles such as soap and shampoo in order to minimize extraneous contact with the target substances.
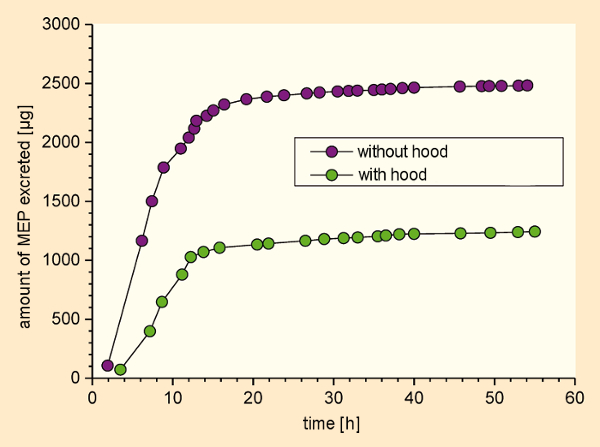
On one of the test days, each experimental subject wore a hood, and inhaled filtered, compressed air (green in Fig. 16). That is, phthalate exposure was allowed exclusively via the skin. On the other day, they breathed the air in the test chamber directly, without any hood (purple in Fig. 16), so that phthalate exposure occurred via both the skin and inhalation. Urine samples were collected during each exposure, and until 48 hours afterward. A total of three metabolites were studied in the samples (one from DEP and two from DnBP). In order to assess uptake from inhalation alone, estimated dermal uptake (based on the day wearing a hood) was subtracted from estimated total uptake on the day without a hood [46].
 |
|
Figure 16. Experimental determination of transdermal accumulation of DEP and DNBP directly from the gas phase (MEP = monoethyl phthalate). |
Exposure of the test subjects to increased gas-phase concentrations of DEP and DnBP via the skin alone led to quantities of metabolites in the urine samples that were only roughly half as great as amounts observed after exposure via both skin and inhalation. This confirmed earlier model calculations that anticipated dermal absorption directly from the air is relevant, provided the substance equilibrated rapidly with the lipid layer of the skin and also migrated rapidly through the skin [47].
Experiment 2
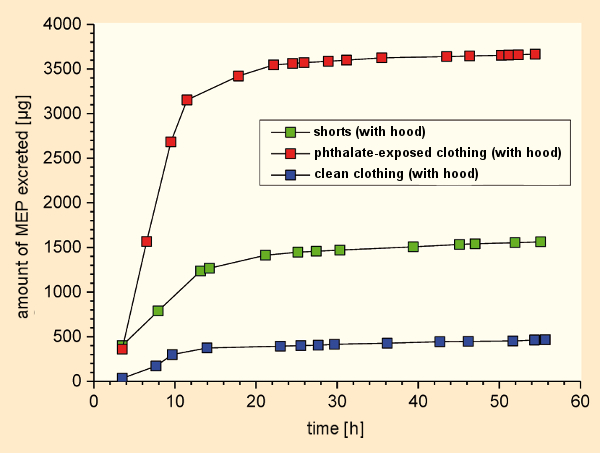
In a second experiment (see Fig. 17), a male subject was exposed to the same conditions as in Experiment 1. But unlike the men clad in shorts in the previous experiment, this subject wore clean cotton clothing (blue in Fig. 17). In yet another experiment, the subject again wore clean clothes, but ones that had previously hung nine days in phthalate-containing air (red in Fig. 17).
 |
|
Figure 17. Experimental determination of transdermal absorption of DEP and DNBP with different clothing (MEP = monoethyl phthalate). |
It turned out that, with freshly-washed clothes, the uptake of phthalates from the air was diminished compared to average accumulations from the air as observed in previous experiments. Wearing clothes that had been stored in room air, however, increased the uptake of phthalates by the test subject relative to unclad conditions [48]. This discovery has definite practical relevance, since many chemical substances used to protect clothing against moth infestation contain active ingredients like pyrethroids.
8. Humans as the Source of Substances
Humans are also a significant source of inorganic and organic compounds, most of them released by exhalation. So far, many hundreds of substances have been identified analytically in exhalates, with respective concentrations and chemical compositions dependent on the individual metabolism. Chief components are acetone, isoprene, methanol, ethanol, and ammonia [49].
After the consumption of alcohol [50], the dominant components are ethanol and acetaldehyde; with ingestion of pectin-containing foods and beverages, one can readily detect the especially water-soluble substance formaldehyde in exhalation gases [51]. Certain diseases are detectable on the basis of organic markers in exhalants. For example, people suffering from diabetes exhibit elevated levels of acetone in their breath [52], and those with liver damage exhale significant amounts of ammonia.
Proton transfer reaction mass spectrometry (PTR-MS) is well suited to the analysis of organic components. This is an especially rapid and sensitive procedure for the real-time determination of trace concentrations in the gas phase [53].
Carbon Dioxide
The most important metabolite is, of course, carbon dioxide. During seated activities, an adult normally exhales 15–20 liters of CO2 per hour. With more strenuous physical activity, the CO2 output may rise to ca. 100 liters per hour.
CO2 output can become problematic with high room occupancy and limited ventilation, as for example in school classes and lecture halls. This leads not only to a rapid increase in the CO2 concentration, but also in both temperature and humidity, all of which is associated with a significant deterioration in the perceived quality of the air [26].
Air in movie theaters also tends to seem “thick”, an observation that scientists at the Max-Planck-Institute for Chemistry in Mainz, Germany, used as encouragement to study air quality via the ventilation system during 108 different film screenings. The question was raised whether diverse scenes might be differentiated on the basis of the chemical makeup of exhaled air.
The study encompassed fourteen films from different genres. The concentration/time profiles for carbon dioxide, as well as quantitative signatures for organic compounds, were recorded at 30-second intervals, and then matched against the respective film scenes. For each film, individual scenes (comic, suspenseful, romantic, etc.) were evaluated separately, with groups of ten people in the audience. Statistically meaningful, distinct chemical signals were established with respect to both humorous and suspenseful scenes.
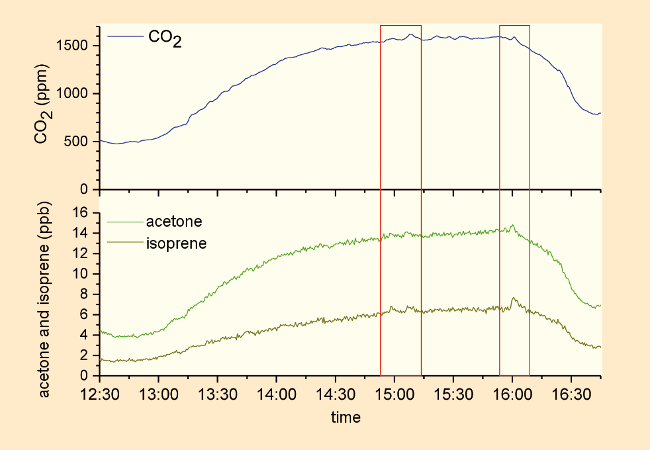
Especially significant was a chemical signature from the film “The Hunger Games – Catching Fire”, which was repeatedly observed with various audiences (see Fig. 18). When the protagonist Katniss Everdeen was fighting for her life, the audience members became distinctly restless, and they breathed more rapidly. Consequently, levels of carbon dioxide, isoprene, and acetone in the air always rose strikingly [54].
 |
|
Figure 18. Time/concentration profiles for carbon dioxide, isoprene, and acetone during a presentation of “The Hunger Games – Catching Fire”. |
9. “Life on Mars?”
This question, posed by David Bowie in the album “Hunky Dory”, should—in accord with the wishes of NASA—become definitively answerable in 2030. A Mars expedition has been in the planning stages for quite some time, and from a technical standpoint, it should be quite feasible. The biggest problem is that any astronauts involved must not only be conveyed to Mars, but also brought back again, and that they must not be at each other’s throats during the long voyage (or perhaps make passes at each other!). In other words, not only must the air quality be kept reasonable, but so must the quality of life generally.
Possible problems with respect to air quality have been especially beautifully thematized in the book and film “The Martians”. Inadvertently left behind on Mars, and with no hope of rescue in the near future, the protagonist Mark Watney faces the challenge of providing himself with breathable air and potable water in his dome. The chemistry he takes advantage of has been especially well presented.
Oxygen
For a source of oxygen, Watney takes advantage of the principle of catalytic reaction at a solid electrolyte (SOXE = Solid Oxide Electrolysis) [55]. With this procedure, in a reversal of processes carried out in fuel cells, carbon dioxide from the Martian atmosphere is reduced to carbon monoxide and oxygen.
For this, the carbon dioxide is passed over a metal-oxide electrode at high temperatures (800–900 °C), resulting in carbon monoxide and the oxygen anion (O2–). The latter migrates through an electrolyte to the anode, where it is oxidized to oxygen gas. To increase the yield of oxygen, the protagonist uses multiple electrolysis cells in a single system. Subsequent separation of O2 from CO2/CO presents no serious problem (see Fig. 19).
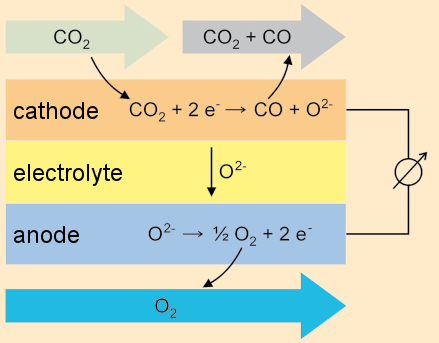 |
|
Figure 19. Production of oxygen using solid oxide electrolysis (SOXE). |
This does not in any sense constitute science fiction, by the way. NASA has already been experimenting—against the background of a future Mars expedition—on converters of precisely this type under the name MOXIE (Mars Oxygen ISRU Experiment), where ISRU stands for “In Situ Resource Utilization”. The Martian atmosphere consists of 95 % CO2, albeit at an atmospheric pressure of only 7–9 mbar. Thus, there would be a need for a pressure increase through compression at the outset [55].
Water
More difficult is the provision of water, which Watney needs not only for drinking, but also for cultivating potatoes. The only suitable chemical source material available to him is the rocket fuel hydrazine. He manages to reduce this compound over a rare metal catalyst below 300 °C to ammonia and nitrogen. Subsequently, in a reversal of the Haber-Bosch process, he prepares a mixture of nitrogen and hydrogen from the ammonia (see Fig. 20). This step requires a certain amount of caution, since in the presence of a heated metal like tungsten or platinum, hydrazine decomposes explosively to nitrogen and ammonia. Subsequently, water is produced by the combustion of hydrogen with oxygen.
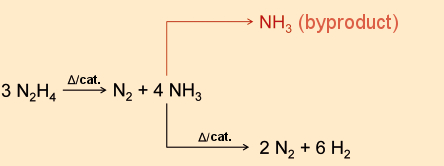 |
|
Figure 20. Production of hydrogen. |
It is difficult to say whether ammonia would present a serious problem for Watney. The maximal allowed work-place concentration of ammonia is 14 mg/m3 (20 ppm) [56], and the odor threshold is ca. 2–3 ppm [57]. Under the given circumstances, he could live (i.e., survive) at an ammonia concentration up to 5 ppm.
A cute marginal note for analytical chemists: in the film, the requisite gas chromatography/mass spectrometry (GC/MS) system is visible in the background.
References
[46] C. J. Weschler et al., Transdermal Uptake of Diethyl Phthalate and Di(n-butyl) Phthalate Directly from Air: Experimental Verification, Environ. Health Perspect. 2015, 123, 928–934. https://doi.org/10.1289/ehp.1409151
[47] C. J. Weschler, W. W. Nazaroff, SVOC exposure indoors: fresh look at dermal pathways, Indoor Air 2012, 22, 356–377. https://doi.org/10.1111/j.1600-0668.2012.00772.x
[48] G. C. Morrison et al., Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs, J. Expos. Sci. Environ. Epidemiol. 2016, 26, 113–118. https://doi.org/10.1038/jes.2015.42
[49] D. Smith et al., Acetone, ammonia and hydrogen cyanide in exhaled breath of several volunteers aged 4–83 years, J. Breath Res. 2007, 1, 014004. https://doi.org/10.1088/1752-7155/1/1/011001
[50] K. Roth, Die Chemie des Katers: Alkohol und seine Folgen (in German), Chem. Unserer Zeit 2007, 41, 46–55. https://doi.org/10.1002/ciuz.200700409
[51] U. Riess et al., Experimental setup and analytical methods for the non-invasive determination of volatile organic compounds, formaldehyde and NOx in exhaled human breath, Anal. Chim. Acta 2010, 669, 53–62. https://doi.org/10.1016/j.aca.2010.04.049
[52] W. Miekisch et al., Diagnostic potential of breath analysis—focus on volatile organic compounds, Clin. Chim. Acta 2004, 347, 25–39. https://doi.org/10.1016/j.cccn.2004.04.023
[53] R. S. Blake et al., Proton-Transfer Reaction Mass Spectrometry, Chem. Rev. 2009, 109, 861–896. https://doi.org/10.1021/cr800364q
[54] J. Williams et al., Cinema audiences reproducibly vary the chemical composition of air during films, by broadcasting scene specific emissions on breath, Sci. Rep. 2016, 6, 25464. https://doi.org/10.1038/srep25464
[55] M. H. Hecht et al., The Mars Oxygen ISRU Experiment (MOXIE), International Workshop on Instrumentation for Planetary Missions, National Aeronautics and Space Administration (NASA), Greenbelt, MD, 2014.
[56] Deutsche Forschungsgemeinschaft (DFG), MAK- und BAT-Werte-Liste 2016 (in German), WILEY-VCH, Weinheim, 2016. ISBN: 978-3-527-34218-1
[57] M. A. M. Smeets et al., Odor and Irritation Thresholds for Ammonia: A Comparison between Static and Dynamic Olfactometry, Chem. Senses 2007, 32, 11–20. https://doi.org/10.1093/chemse/bjl031
The article has been published in German as:
- The Air that I Breathe – Der Innenraum als chemischer Reaktor,
Tunga Salthammer,
Chem. unserer Zeit 2017.
https://doi.org/10.1002/ciuz.201700779
and was translated by W. E. Russey.
The Air that I Breathe – Part 1
Chemistry of indoor air is a relatively new, diverse and interdisciplinary field surrounding us every day
The Air that I Breathe – Part 2
How dangerous are my household appliances?
The Air that I Breathe – Part 3
Emissions of e-cigarettes and other drugs
The Air that I Breathe – Part 4
Man as a source of substances, life on Mars, and what to learn from movies
The Air that I Breathe – Part 5
How does climate change affect indoor air quality?
See similar articles by Klaus Roth published in ChemViews Magazine



