Anticancer Drugs
Killing malignant mitochondria is one of the most promising approaches in the development of new anticancer drugs. Scientists from the UK have synthesized a copper-containing peptide that is readily taken up by mitochondria in breast cancer stem cells, where it effectively induces apoptosis. The study also highlights the powerful therapeutic potential of the metallopeptides.
Mitochondria are the power factories of the cells and the central “node” of cell death induction. If their mitochondria are taken off, cells will die through apoptosis. Cancer cells, which have an increased metabolism, not only contain more mitochondria than healthy cells, but also different ones, structurally and functionally. The distinctive features and their decisive role in cell metabolism have made malignant mitochondria a prominent target for new therapeutic compounds. Bio-inorganic chemist Kogularamanan Suntharalingam and his group at King’s College, London, UK, explore how mitochondria killing agents can be inserted into the organelles and what damage can be achieved.
Killing Mitochondria
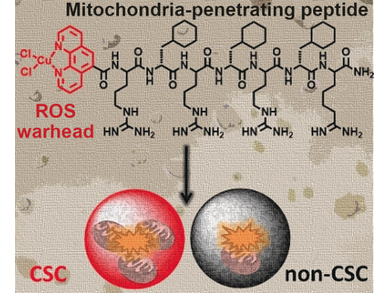
Killing mitochondria could be accomplished, for example, by introducing agents to generate reactive oxygen species (ROS). These reactive compounds interfere with several pathways of the mitochondrial metabolism, having maximal impact on the organelle’s function. Suntharalingam’s group recently proposed the organometallic compound copper(II) phenanthroline as a potent ROS generator with an especially deadly potential for cancer stem cells. However, this special agent must be delivered to its address and shuffled over the outer mitochondrial membrane.
A solution would be to make a parcel, for example, by tethering it to a membrane-soluble peptide specific for mitochondria. “Attachment of mitochondrial-penetrating peptides also enables selective and efficient delivery to mitochondria,” the researchers proposed.
Copper-Containing Metallopeptide
The scientists, including Nicola O’Reilly’s peptide chemistry group at The Francis Crick Institute, London, UK, prepared the deadly parcel made of a copper-containing metallopeptide by covalently linking the phenanthroline copper(II) complex to an established mitochondria-penetrating peptide. The tests were performed with two breast cancer cell lines, one the bulk cancer cell line and the other one a cell line enriched with cancer stem cells, which constitute the heart of the cancer and often dodge conventional therapies.
The results were impressive: The researchers observed a dose-dependent viability loss of up to 100 % of the cells, mitochondrial membrane disintegration, drug intake into the mitochondria, ROS generation, and impairment of the mitochondrial metabolism. The drug also affected the cancer stem cells more than the normal cell line, which was explained by their higher content of mitochondria.
According to the researchers, this study highlights the potential of the metallopeptides as both delivery and killing agents, especially for cancer stem cells.
- A Copper(II) Phenanthroline Metallopeptide That Targets and Disrupts Mitochondrial Function in Breast Cancer Stem Cells,
Kristine Laws, Ganka Bineva-Todd, Arvin Eskandari, Chunxin Lu, Nicola O’Reilly, Kogularamanan Suntharalingam,
Angew. Chem. Int. Ed. 2017.
https://doi.org/10.1002/anie.201710910