Portable electronics need batteries with ever larger capacities, and the commonly used lithium-ion batteries struggle to keep up. Replacing the graphite anodes in such batteries with lithium metal could theoretically raise the capacity significantly. However, lithium metal forms dendrites during charge/discharge cycles, which can cause dangerous short circuits. In addition, the uneven deposition of Li can cause mechanical failure of the electrode and the accumulation of inactive metal.
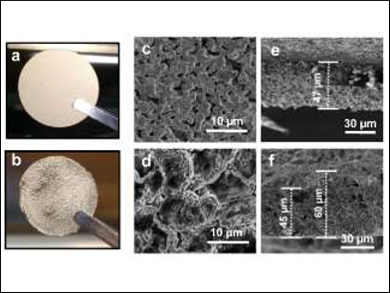
Wu Xu, Ji-Guang Zhang, Pacific Northwest National Laboratory, Richland, WA, USA, and colleagues have embedded lithium in a porous nickel electrode to produce an anode material with improved properties. The team prepared thin porous nickel sheets (pictured top left) by combing nickel oxide particles, pore forming agents, and additives in a ball-milling process and casting, drying, and reducing the mixture. Then they used electrodeposition to fill the pores of the Ni structure with Li metal (product pictured bottom left).
The synthesized anode material has a significantly improved cycling stability compared with pure Li metal. The researchers attribute this effect to a suppression of the formation of inactive lithium and to improved Li ion transport. The porous structure helps to preserve a uniform Li surface during charge/discharge cycles and hinders the growth of dendrites, which improves safety.
- Enhanced Stability of Lithium Metal Anode by 3D Porous Nickel Substrate,
Lu Yu, Nathan L. Canfield, Shuru Chen, HongKyung Lee, Xiaodi Ren, Mark H. Engelhard, Qiuyan Li, Jun Liu, Wu Xu, Ji-Guang Zhang,
ChemElectroChem 2018.
https://doi.org/10.1002/celc.201701250