CO2-Free Hydrogen Storage Cycle
Hydrogen is under consideration as a promising energy carrier for a future sustainable energy economy. However, practicable solutions for the easy and safe storage of hydrogen are still being sought. Despite some progress, no generally applicable solutions that meet the requirements of industry have been found to date. In the journal Angewandte Chemie Matthias Beller and his team at the Leibnitz Institute for Catalysis, Rostock, Germany, have now introduced a new approach to hydrogen storage that is based on simple salts of formic acid and carbonic acid.
Practical hydrogen storage materials must take up and give off hydrogen at standard pressure and room temperature, accommodate a large amount of hydrogen in as little space as possible, and release it rapidly and on-demand. Metal hydride tanks store hydrogen in a relatively manageable volume but are very heavy and expensive, as well as operating only at 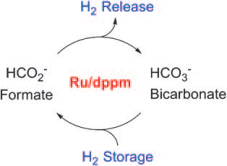 high temperatures or far too slowly. In addition to organic hydrogen storage materials, such as methane and methanol, researchers have been interested in formic acid (HCO2H) and its salts, known as formates, for the generation of hydrogen. A fundamental problem with the use of these storage materials is the separation of the carbon dioxide formed when the hydrogen is released. The team from Rostock has now successfully used a special ruthenium catalyst that catalyzes both the release and uptake of hydrogen to establish a reversible, CO2-free hydrogen storage cycle. In this system, hydrogen is released from nontoxic formates and the resulting CO2 captured in the form of bicarbonates. Bicarbonates are a component of many natural stones and are also commonly used as baking powder or sherbet (sodium bicarbonate, NaHCO3).
high temperatures or far too slowly. In addition to organic hydrogen storage materials, such as methane and methanol, researchers have been interested in formic acid (HCO2H) and its salts, known as formates, for the generation of hydrogen. A fundamental problem with the use of these storage materials is the separation of the carbon dioxide formed when the hydrogen is released. The team from Rostock has now successfully used a special ruthenium catalyst that catalyzes both the release and uptake of hydrogen to establish a reversible, CO2-free hydrogen storage cycle. In this system, hydrogen is released from nontoxic formates and the resulting CO2 captured in the form of bicarbonates. Bicarbonates are a component of many natural stones and are also commonly used as baking powder or sherbet (sodium bicarbonate, NaHCO3).
Many Advantages
“Our new concept has a number of advantages,” says Beller, “in comparison to CO2, solid bicarbonate is easy to handle and is very soluble in water. The resulting bicarbonate solution can be catalytically converted to a formate solution under much milder conditions than those required for the reactions to form methane or methanol.” In addition, the harmless solid could easily be stored and transported. Retrieval of the hydrogen occurs at room temperature or even lower. Says Beller, “Most important is that a closed carbon cycle is now possible because the resulting bicarbonate can simply be loaded up with hydrogen again.”
- CO2-“Neutral” Hydrogen Storage Based on Bicarbonates and Formates
A. Boddien, F. Gärtner, C. Federsel, P. Sponholz, D. Mellmann, R. Jackstell, H. Junge, M. Beller,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201101995




