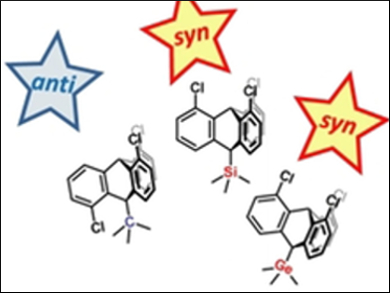
Triptycene is an aromatic hydrocarbon and one of only a few rigid organic frameworks with D3h symmetry and no (Lewis-basic) heteroatoms. This symmetry and the rigidity of triptycenes (examples pictured) make them useful for both fundamental and applied chemical research. For example, substituted triptycenes are commonly used in molecular machines, crystal engineering, as rigid spacers in palladium complexes for cross-coupling reactions, and as building blocks for organic macromolecules, polymers, and ionic liquids. However, 1,8,13-trisubstituted triptycenes (also known as syn-triptycenes) remain difficult to synthesize.
Norbert W. Mitzel, University of Bielefeld, Germany, and colleagues have found that reactions between substituted 1,8-dichloroanthracenes and ortho-chloroaryne afford mixtures of syn-tryptycenes and 1,8,16-trichlorotriptycenes (anti-tryptycenes). The syn:anti ratio is shown to be controlled by the substituents in position 10 of the 1,8-dichloroanthracenes, with SiMe3 and GeMe3 leading to the preferred formation of the syn-isomer and CMe3 exclusively yielding the anti-isomer.
The products were characterized by using NMR spectroscopy, mass spectrometry (MS), and X-ray diffraction. The results could be a key first step towards understanding the multiple factors that determine the regioselectivity of these reactions, which is needed to realize full steric control over triptycenes.
- Regiochemical Control in Triptycene Formation—An Exercise in Subtle Balancing Multiple Factors,
Jan-Hendrik Lamm, Yury V. Vishnevskiy, Eric Ziemann, Beate Neumann, Hans-Georg Stammler, Norbert W. Mitzel,
ChemistryOpen 2018, 7, 111–114.
https://doi.org/10.1002/open.201700196
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


