Changes in the metal composition of organometallic compounds can have subtle effects on the nature of metal bonds. On the one hand, metal ions in a chain can be linked by single or even multiple covalent bonds, a situation that is typical of many organometallic clusters. On the other hand, the electrons may be largely confined to individual metal ions, communicating only weakly through exchange coupling.
The theoretical models that have been developed to treat these two limits are rather different: molecular orbital (MO) theory is the natural choice for covalent bonding, whereas the language of valence bond (VB) theory is better suited to exchange-coupling. This, however, ignores the reality that the two extremes are simply the limits of a continuum of intermediate situations.
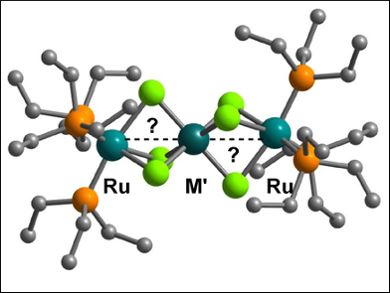
John E. McGrady, University of Oxford, UK, and colleagues have studied the properties of tri-metallic chains containing heavy transition metal ions, namely [(PEt3)3RuCl3M′Cl3Ru(PEt3)3]+ (pictured, M′ = Rh,Ir). X-ray crystallography and spectroelectrochemistry were used to investigate the nature of the interaction between the metal ions across a range of oxidation states.
The team concluded that the studied compounds fall between the traditional covalent/exchange-coupled limits. Density functional theory (DFT) and other quantum chemical calculations showed that a subtle balance between orbital overlap and electron-electron repulsion controls the compounds’ physical properties.
- Redox-Dependent Metal−Metal Bonding in Trinuclear Metal Chains: Probing the Transition from Covalent Bonding to Exchange Coupling,
Mohammed Obies, Nicholas R. Perkins, Vaida Arcisauskaite, Graham A. Heath, Alison J. Edwards, John E. McGrady,
Chem. Eur. J. 2017.
https://doi.org/10.1002/chem.201704727