Metal bis(porphyrinato) double-decker complexes have been used for the construction of functional molecules such as molecular machines and single-molecule magnets (SMMs). One of the important features of the complexes is redox-multistability. To understand differences in the properties of the various oxidation forms, understanding their exact structure is important.
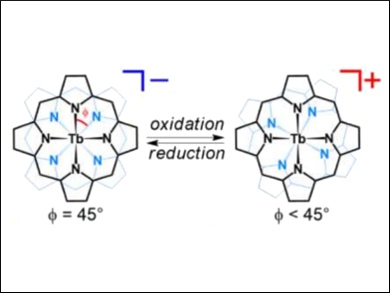
Ken-ichi Yamashita, Takuji Ogawa, and colleagues, Osaka University, Japan, have found a redox-driven symmetry change for terbium(III) bis(porphyrinato) double-decker complexes of the type [TbIII(por)2]n (n = –1,0,+1) that involves the azimuthal rotation of the porphyrin macrocycles. The anionic form (n = –1) has a symmetric structure with φ = 45 ° (φ = the azimuthal rotation angle between the two porphyrin macrocycles). Upon oxidation, φ becomes smaller (pictured). This change is induced by a steric effect on the substituents and/or an electronic effect on the porphyrin macrocycles.
The revealed structures could contribute not only to further understanding the relationship between the complexes’ structure and their SMM properties, but could also help with the design of redox-driven advanced materials such as molecular machines.
- Redox-Driven Symmetry Change for Terbium(III) Bis(porphyrinato) Double-Decker Complexes by the Azimuthal Rotation of the Porphyrin Macrocycles,
Ken-ichi Yamashita, Takayo Yamanaka, Naoya Sakata, Takuji Ogawa,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800324