Metal-oxido compounds are widespread both in the earth’s crust and in biology, for example, in metalloenzymes. Metal oxides are highly stable and often represent the “thermodynamic sink” after reaction with molecular O2. It is, thus, highly desirable to find ways to increase their reactivity.
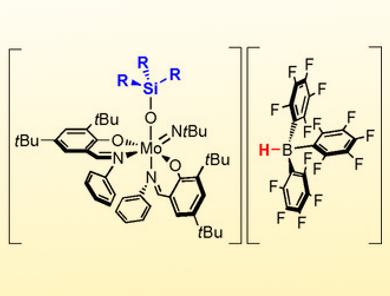
Nadia Mösch-Zanetti and colleagues, University of Graz, Austria, have used the Lewis basicity of a molybdenum oxide ([MoO(NtBu)L2]) and added the strong Lewis acid B(C6F5)3 to induce reactivity similar to that of frustrated Lewis pairs (FLP). This resulted in the Lewis adduct [Mo{OB(C6F5)3}(NtBu)L2] featuring reversible B−O bonding in solution. The molybdenum oxido based Lewis adduct reacts with tertiary silanes to form highly unusual ion pairs. The frustrated Lewis pair (FLP)‐like reactivity is reflected by the ability to heterolytically cleave Si−H bonds. The reaction of the ion pairs with benzaldehyde allowed for the regeneration of the Lewis adduct by hydrosilylation of the benzaldehyde.
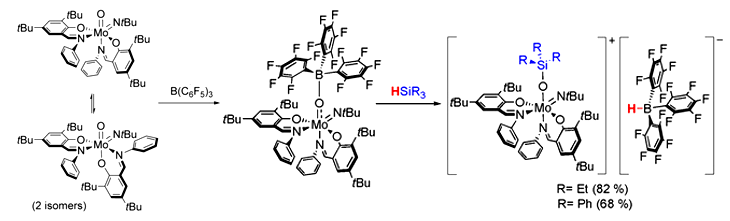
This is a rare example of FLP‐like reactivity involving the widespread transition metal oxido functionality. In addition, it gives access to high valent molybdenum species with unique spectroscopic and electronic properties of potential interest to a broad field of chemistry. Furthermore, it represents a rare instance of a Lewis acid mediated activation of the oxido ligand. According to the researchers, their findings allow to induce FLP-like reactivity next to redox-active metal centers in general.
- Heterolytic Si–H Bond Cleavage at a Molybdenum-Oxido-Based Lewis Pair,
Niklas Zwettler, Simon P. Walg, Ferdinand Belaj, Nadia C. Mösch-Zanetti,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201800226
Correction (May 25, 2018)
The article originally gave an incorrect formula for the molybdenum oxide.