Electrochemical water splitting is a sustainable way of producing hydrogen. Because the slow kinetics of oxygen-evolution reactions (OERs) hinder the wider use of this technology, new, effective catalysts for OER are needed. Transition metal oxides, (oxy)hydroxides, and their derivatives are promising materials due to their tunability, low cost, and stability. Binary and ternary metal-based hydroxides are of particular interest in this context due to synergistic effects between the metals.
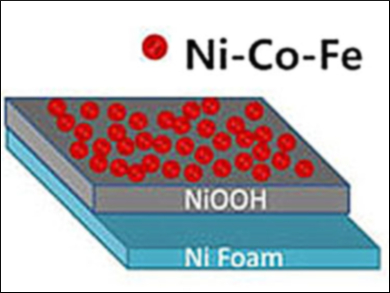
Xiujuan Wu and colleagues, Dalian University of Technology, China, have prepared a ternary NiCoFe hydroxide anode which is remarkably durable and effective for OER. In the first step, a nickel foam is cleaned with phosphoric acid to remove surface nickel oxide species. The nickel foam is placed in a solution of cobalt(II) chloride, iron(III) sulfate, and nickel(II) acetate for metal plating. Anodic deposition, followed by activation of the deposited material by cyclic voltammetry, results in a homogeneous coverage of the nickel foam with nanosheets of NiCoFe hydroxides.
The researchers found that a NiOOH interlayer forms on substrates pretreated with phosphoric acid, but not on substrates cleaned with HCl. This electrically conductive interlayer increases the electrocatalytic activity of the prepared anode in OER. Overpotentials of 223 and 254 mV yielded current densities of 10 and 100 mA cm–2, respectively, in 1 M KOH. Fast electrochemical kinetics and the stability of the anode were also confirmed. This research points to a new strategy for simplifying and optimizing the fabrication of nanostructured electrocatalysts for water oxidation.
- Facile Synthesis of a Ternary Metal Hydroxide with Acid Treatment as an Effective and Durable Electrocatalyst in Water Oxidation,
Xiujuan Wu, Tongyu Xing, Yimeng Zhao, Husileng Lee, Peili Zhang,
ChemPlusChem 2018.
https://doi.org/10.1002/cplu.201800053This article is part of the ChemPlusChem special issue “Professor Leone Spiccia Memorial Issue“,
ChemPlusChem 2018, 83 (7), which is free to view through July 2018.