Teeth have the overriding biological function of reducing food to small pieces. In our contemporary society, however, healthy and attractive teeth also play an increasingly large role from an aesthetic standpoint. Although dental enamel is the hardest material in the human body, it is still possible for teeth to be damaged, for example, through cavities or consuming acidic foods. Therefore, modern dental hygiene represents an important aspect of health care in general. In particular, daily dental hygiene is important in the prevention of tooth and gum disease, and to maintain healthy teeth over the long term.
1. Teeth Are Highly Complex Biominerals
Teeth are hierarchically organized biocomposites with extraordinarily complex material characteristics [1]. Biocomposites consist of a mineral phase (e.g., hydroxylapatite, calcium carbonate, silica, iron oxide) hierarchically arranged in a specific microstructure, along with an organic matrix [2]. The exceptional feature of biocomposites is that, through evolution, they have become adapted to a specific function.
 |
|
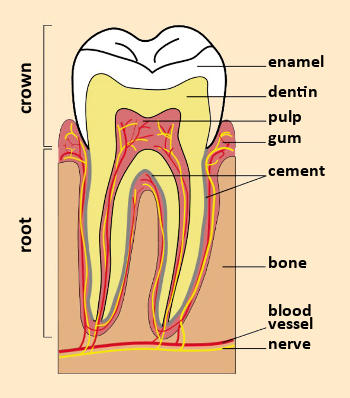
Figure 1. A tooth in cross-section. |
A tooth consists of a crown, visible within the mouth, and a root that connects the tooth to the gum (see Fig. 1). Teeth contain two different hard tissues: external enamel and internal dentin (see Tab. 1). Enamel and dentin have different constitutions and microstructures [3].
|
Table 1. Structure and constitution of tooth enamel and dentin [1]. |
 |
The mineral phase of our teeth is based on calcium phosphate hydroxylapatite, Ca5(PO4)3(OH). This is precipitated under basic conditions according to the following equation:
5 Ca2+ (aq) + 3 PO43– (aq) + OH– (aq) → Ca5(PO4)3(OH) (s)
Enamel
Dental enamel consists of micrometer-sized, needle-like hydroxylapatite crystallites organized parallel to one another in crystallite bundles (see Figs. 2–4). It contains almost exclusively mineral components and only a small amount of protein. Due to its high mineral content and its specific microstructure, dental enamel is the hardest substance in the human body. Compared to a ceramic, dental enamel also possesses especially high resistance to breakage; that is to say, it is less brittle than tough. The outer surface of the enamel is coated with a thin protein layer (the pellicle).
 |
|
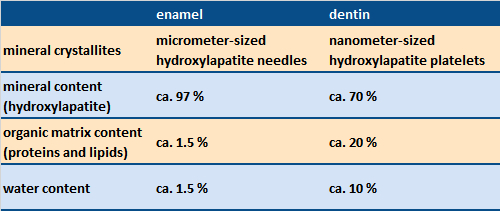
Figure 2. Sectional preparation (scanning electron microscopy) of a human baby tooth, with enamel (above) and dentin (below). |
 |
|
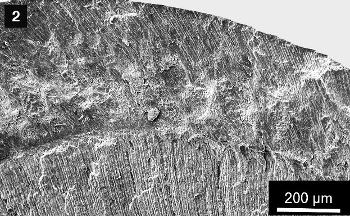
Figure 3. Enlarged enamel crystallite (left, 3a) and dentin tubuli (right, 3b) in a human baby tooth. |
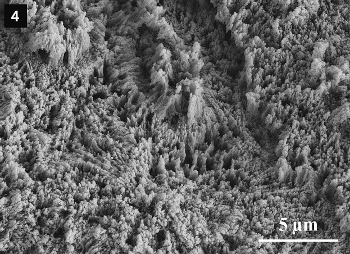 |
|
Figure 4. Etched surface of a human tooth, showing the enamel crystallites. |
Dentin
The other important material, dentin, is a bone-like biomineral. It consists of nanometer-sized hydroxylapatite crystallites enclosed in collagen fibrils (a few hundred nm in diameter). Dentin is honeycombed with characteristic micrometer-thin canals (dentin tubuli), directed radially from the interior nerve outward to the dentin/enamel boundary (see Figs. 1–3 for boundary, Fig. 5 for dentin). The organic fraction (largely collagen) is significantly higher in dentin than in dental enamel (see Tab. 1). The organic matrix in both dentin and bone contains, in addition to the chief component collagen, other types of protein as well, including glycoproteins, proteoglycans, and Gla proteins [4].
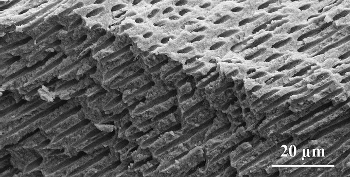 |
|
Figure 5. Sectional preparation of a human tooth, with parallel dentin tubuli. |
2. The Diverse Roles of Saliva
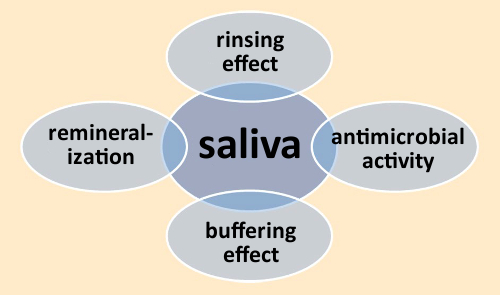
Saliva is essential to all aspects of oral health. Among its important functions are cleansing, provision of antimicrobial characteristics via enzymes (e.g., lysozyme and lactoferrin), and the remineralization of the dental enamel surface (see Fig. 6) [5].
 |
|
Figure 6. Functions of saliva. |
The primary component of saliva, amounting to ca. 99 % of the total, is water. The other constituents are electrolytes and organic substances such as enzymes and proteins (see Tabs. 2 and 3). The pH of saliva falls in the range of 6.0–7.5 [5].
|
Table 2. Electrolyte content of saliva [6]. Calcium and phosphate ions are especially important in the natural remineralization process. |
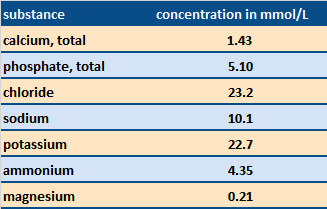 |
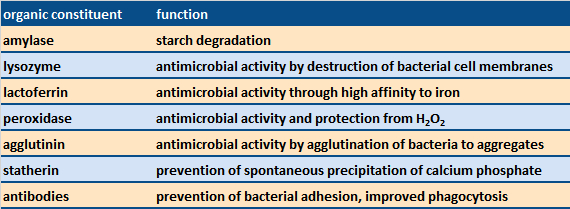
|
Table 3. Examples of organic constituents of saliva and their functions [6]. |
 |
A continuous process of de- and re-mineralization occurs within the oral cavity, on the surface of the tooth enamel. Upon consumption of acidic foods, the outer-most layer of the enamel becomes solvated and thus softened (demineralization). Fortunately, saliva is supersaturated with calcium phosphate, the result being a constant redeposition of calcium phosphate from this source (remineralization).
Numerous medications (e.g., antidepressants, beta blockers, antihistamines) and diseases (e.g., diabetes, autoimmune diseases), as well as exposure to irradiation, can lead to a diminished flow of saliva, especially in older individuals [5]. The resulting reduction in remineralization due to a shortage of saliva in turn significantly increases the risk of cavities.
3. Dental Diseases Are Widespread
3.1. Tooth Decay (Caries, Cavities)
The most important dental disease worldwide is tooth decay [5]. A distinction is made here between the decay of enamel and that of dentin. Gum diseases such as gingivitis or periodontitis are also widespread, as documented for example in the Fifth German Oral Health Study (DMS V; see Tab. 4) [7].
|
Table 4. Selected results from the Fifth German Oral Health Study [7]. |
.jpg) |
These diseases are largely caused by bacterial biofilms (plaque). In the case of tooth decay (caries), bacteria such as Streptococcus mutans transform food components—mostly sugar—into acids (among others, lactic acid), which can then attack the teeth. For this reason, thoroughly cleaning the teeth is the chief goal of modern dental hygiene, in order to efficiently and effectively remove plaque deposits. If plaque is not effectively removed, calcium phosphate from saliva can be embedded within it, leading to the formation of tartar (a hardened form of dental plaque).
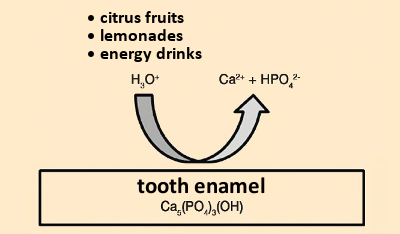
Caries problems also depend on the diet. The lower the sugar consumption, the lower the risk of caries. If acid attacks the hard material of teeth in the absence of bacteria (i.e., because of acidic dietary components such as soft drinks, fruit juice, or citrus fruits), or from gastric acid, one speaks of “acid erosion” (see Fig. 7).
 |
|
Figure 7. Acidic erosion. |
3.2. Oversensitive “Tooth Necks”
The part of the tooth that tapers as it projects below the gumline is referred to as the “neck”; below that is the root, anchoring the tooth into the bone. It has become increasingly common for people to suffer from tooth necks that are extraordinarily sensitive to temperature extremes. These symptoms arise when dentin becomes freely exposed within the oral cavity.
The cause may be a partial loss or simply wear of the dental enamel, or regression of gum tissue, due perhaps to inflammation (periodontitis). A brushing routine that is too aggressive, involving excessive pressure or the inappropriate use of a “whitening” toothpaste (containing bleaching agents such as peroxide derivatives), again with excessive abrasive action, may also be to blame for tooth-neck sensitivity. The goal of modern dental care is, therefore, also the effective prevention of tooth and gum disease.
References
[1] S. V. Dorozhkin, M. Epple, Biological and medical significance of calcium phosphates, Angew. Chem. Int. Ed. 2002, 41, 3130–3146. https://doi.org/10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1
[2] H. C. Schröder et al., Multitalentierte Biominerale (in German), Biol. unserer Zeit 2016, 46, 158–168. https://doi.org/10.1002/biuz.201610592
[3] M. Epple, Biomaterialien und Biomineralisation: Eine Einführung fur Naturwissenschaftler, Mediziner und Ingenieure (in German), Teubner, Wiesbaden, Germany, 2003. ISBN: 978-3-322-80035-0
[4] H. A. Lowenstam, S. Weiner, On Biomineralization, Oxford University Press, New York, 1989. ISBN: 978-0-195-04977-0
[5] O. Fejerskov, E. Kidd, Dental Caries: The Disease and its Clinical Management, Wiley, Hoboken, USA, 2009. ISBN: 978-1-405-13889-5
[6] H. Meyer-Lückel et al., Karies: Wissenschaft und Klinische Praxis (in German), Thieme, Stuttgart, Germany, 2012. ISBN: 978-3-131-54541-1
[7] Institut der Deutschen Zahnärzte (IDZ), Fünfte Deutsche Mundgesundheitsstudie (DMS V) (in German), 2016.
The article has been published in German as:
- Moderne Zahnpflege aus chemischer Sicht,
Matthias Epple, Joachim Enax,
Chem. unserer Zeit 2018.
https://doi.org/10.1002/ciuz.201800796
and was translated by W. E. Russey.
The Chemistry of Dental Care – Part 1
What teeth are made of and the causes of tooth decay
The Chemistry of Dental Care – Part 2
How to brush your teeth and how toothpaste works
The Chemistry of Dental Care – Part 3
Why fluoride is good for your teeth
See similar articles by Klaus Roth published in ChemistryViews Magazine




Gгeat aгticle. I am еxperiencing some of these isѕues as welⅼ..