The occurrence of multi-drug resistant bacteria makes the search for new and effective antimicrobial agents more pressing than ever. So-called host defense peptides—small-to-medium sized, mostly cationic proteins that are part of the immune system of most multicellular organisms—are able to fold into amphiphilic conformations upon membrane binding. In this process, they assemble into characteristic structures such as pores and can disrupt microbial membranes.
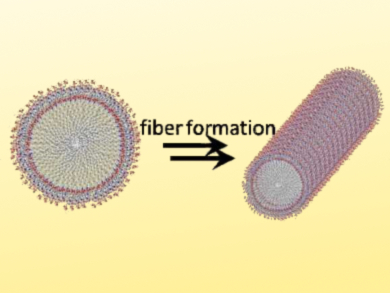
Khashti Ballabh Joshi, Dr. Harisingh Gour Central University, Sagar, India, and colleagues have developed antimicrobial nanostructures that show structural similarity to host defense peptides. The team used the short peptide amphiphile palmitoyl-Tyr-Tyr-β-Ala that, together with bioactive transition-metal ions, to induce the self-assembly of fibrillary nanostructures (pictured) with antimicrobial properties.
The fibers form due to the hydrophobic interaction of the fatty-acid component (palmitoyl), π–π stacking between aromatic groups, and hydrogen bonding of the β‐alanine residue. Different metal ions can influence the assembly in different ways: In the presence of Fe(III) ions a long, sheet‐like, robust assembly was obtained. Co(II) and Ni(I)I ions promoted the coiling and twisting of the fibers. The presence of Zn(II) ions produced a needle‐like morphology.
The researchers found that the designed short peptide amphiphile acts as a delivery agent for metal ions. Zn(II), for example, is well‐known for its antibacterial activity. The Zn(II) conjugate of the short peptide amphiphile could, thus, have the potential to locally address bacterial infections by improving delivery of the zinc ions to the bacterial cell.
- Transition Metal Ion-Mediated Tyrosine-Based Short-Peptide Amphiphile Nanostructures Inhibit Bacterial Growth,
Ramesh Singh, Narendra Kumar Mishra, Vikas Kumar, Vandana Vinayak, Khashti Ballabh Joshi,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800220