Hydrogen is a promising energy carrier for a carbon-free economy. One of the ways to produce renewable hydrogen is using photovoltaic (PV) electricity to electrolyze water. This can be achieved with a proton-exchange membrane water electrolyzer (PV-PEM). However, the PEM creates a locally acidic environment. The only commercially available oxygen evolution catalyst that works under these conditions is IrOx, which is based on one of the scarcest elements in the Earth’s crust. Hence, the development of alternative catalysts based on earth-abundant elements would be useful.
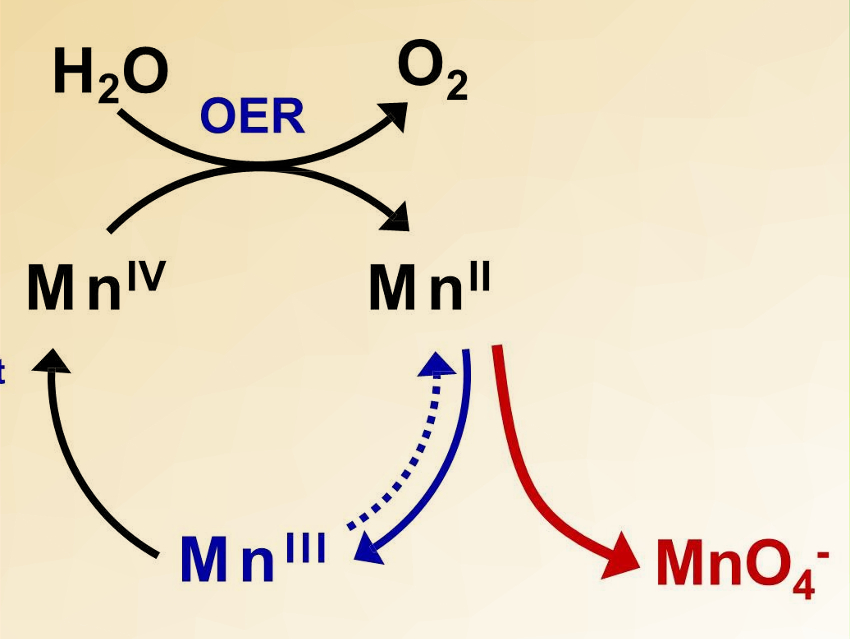
Ryuhei Nakamura, RIKEN, Japan, Hongxian Han, Chinese Academy of Sciences, China, and colleagues have discovered that γ-MnO2 can catalyze water oxidation under acidic conditions. The team prepared γ‐MnO2 directly on fluorine‐doped tin oxide (FTO) or carbon‐based substrates by thermal decomposition of manganese nitrate in air at 220 °C.
The catalyst is stable for over 8,000 hours with a stable potential window between 1.6 V and 1.75 V. A voltage efficiency of 70 % was achieved in a PEM electrolyzer. This work demonstrates that it is possible to develop acid-stable earth-abundant OER catalysts to replace IrOx, making PV-PEM a viable means for renewable hydrogen production.
- Stable Potential Windows for Long-Term Electrocatalysis by Manganese Oxides Under Acidic Conditions,
Ailong Li, Hideshi Ooka, Nadège Bonnet, Toru Hayashi, Yimeng Sun, Qike Jiang, Can Li, Hongxian Han, Ryuhei Nakamura,
Angew. Chem. Int. Ed. 2019, 58, 5054–5058.
https://doi.org/10.1002/anie.201813361