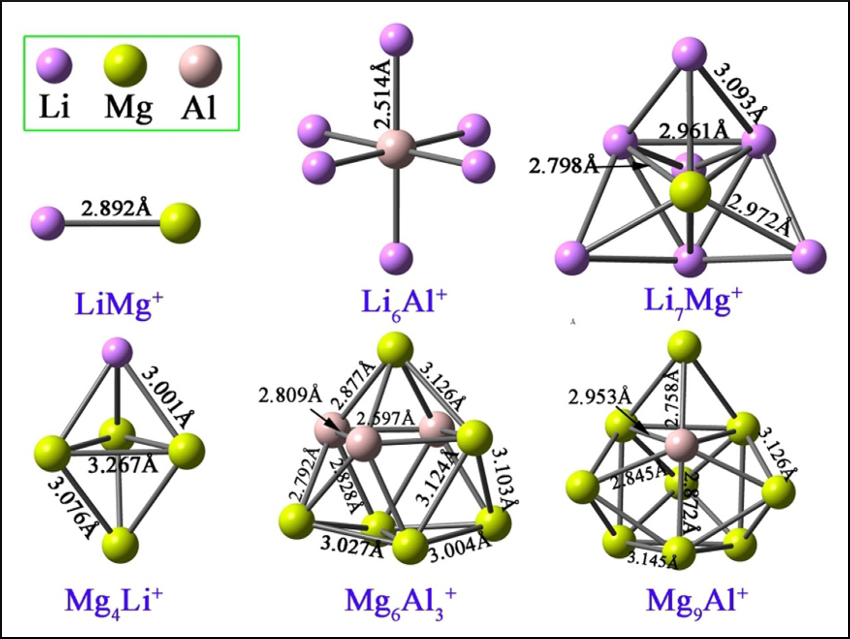
Superalkalis are polyatomic clusters which have even lower ionization energies (IEs) than those of alkali metal atoms. They can, e.g., activate small molecules or be used in nanoscale materials. The development of new strategies to obtain superalkali species would, thus, be useful.
Wei-Ming Sun, Fujian Medical University, Fuzhou, China, and colleagues have used the jellium model, a simple quantum-chemical model, as a guide to rationally design bimetallic superalkali cations using density functional theory (DFT). The researchers proposed a series of bimetallic cationic clusters (examples pictured) with 2, 8, 20, or 40 valence electrons and Li, Mg, and Al as atomic building blocks. The corresponding neutral clusters tend to lose one valence electron to achieve closed-shell states in the jellium model. Thus, the cations have electron affinities that are much lower than the IEs of alkali metal atoms, which indicates that the cationic clusters are superalkalis.
Using further computations, the team also found that the designed superalkalis could be used as reductants to activate CO2 and N2 molecules or as stable building blocks to assemble ionic superatom compounds. This study could provide an effective method to obtain various metallic superatoms on the basis of the simple jellium rule.
- On the Possibility of Using the Jellium Model as a Guide To Design Bimetallic Superalkali Cations,
Wei-Ming Sun, Xiao-Ling Zhang, Kai-Yun Pan, Jing-Hua Chen, Di Wu, Chun-Yan Li, Ying Li, Zhi-Ru Li,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201806194