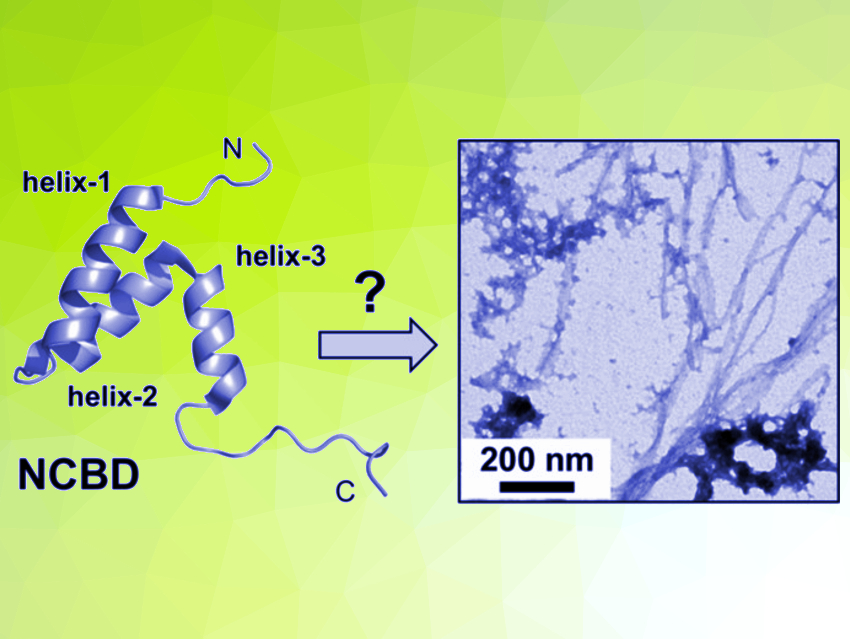
Protein misfolding into insoluble amyloid fibers is associated, e.g., with Alzheimer’s and Parkinson’s disease. Many proteins have a propensity to misfold and self-assemble into amyloid fibers. Protein misfolding is a highly complex process. The misfolding and aggregation into amyloids can start, e.g., from a fully folded protein or from intrinsically disordered sequences. Discovering such alternative folding pathways for proteins can be challenging.
Vladimir Torbeev, University of Strasbourg, France, and colleagues have shown that the nuclear coactivator binding domain (NCBD) of CREB-binding protein (CBP) can form amyloids. CBP is an important protein for regulating gene transcription. The team investigated the aggregation of NCBD in the presence of the mirror-image protein D-NCBD. Such mirror-image proteins enable new modes of molecular recognition and self-assembly; racemic protein mixtures, for example, crystallize more readily than single enantiomers. The D-NCBD enantiomer was prepared by chemical synthesis analogously to L‐NCBD, using commercially available D-amino acid building blocks.
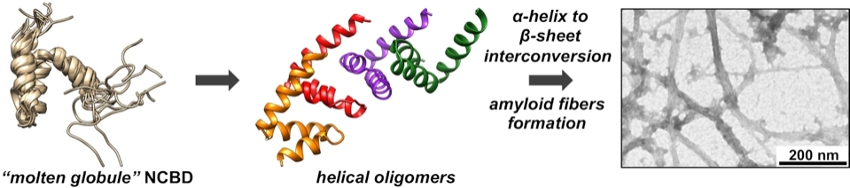
NCBD has properties of a “molten globule”, which shows some elements of secondary structure but lacks the well-defined tertiary structure typical for folded proteins (pictured below on the left). The researchers used solubility experiments, attenuated total reflection Fourier transform infrared (ATR‐FTIR) spectroscopy, and electrospray ionization mass spectrometry (ESI‐MS) to observe the aggregation process of NCBD. They found that the initial steps of protein misfolding in NCBD are driven by intermolecular interactions of its N‐terminal α‐helix, bringing multiple NCBD molecules into contact. These oligomers then undergo a slow transformation to β‐sheet‐containing aggregates (pictured below on the right). The addition of D-NCBD promoted self‐assembly into amyloid precipitates, presumably due to the formation of thermodynamically more stable racemic β‐sheet structures.
- Aggregation and Amyloidogenicity of the Nuclear Coactivator Binding Domain of CREB‐Binding Protein,
Ana Maria Garcia, Christophe Giorgiutti, Youssef El Khoury, Valentin Bauer, Coralie Spiegelhalter, Emmanuelle Leize‐Wagner, Petra Hellwig, Noelle Potier, Vladimir Torbeev,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202001847