As the sole artificial means to catalyze the reduction of atmospheric N2 to NH3, the Haber-Bosch process carries an enormous industrial and agricultural importance. Yet, despite being developed nearly a century ago and its immense economic and environmental cost, no cleaner or more efficient alternative to this process has been found.
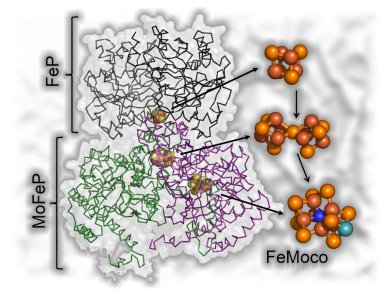
Biological nitrogen fixation catalyzed by nitrogenase is an enticing place to look for inspiration for an alternative catalyst. The catalytic mechanism of this enzyme is poorly understood, because it relies upon ATP hydrolysis and protein interactions to coordinate electron transfer and substrate activation, creating a complex mixture of redox intermediates during turnover. Recently, new approaches have been presented to circumvent this problem. One such possibility is the use of covalently attached photosensitizers to directly transfer electrons to the enzyme active site, thereby uncoupling substrate reduction from ATP hydrolysis and allowing the population of discrete reaction intermediates for structural or spectroscopic investigation.
L. E. Roth and F. A. Tezcan, University of California, San Diego, USA, describe how biological nitrogen fixation, catalyzed by nitrogenase, could rival the Haber–Bosch process and give an overview of this important area of biocatalysis research in their concept article.
- Light-Driven Uncoupling of Nitrogenase Catalysis from ATP Hydrolysis
L. E. Roth and F. A. Tezcan
ChemCatChem 2011.
DOI: 10.1002/cctc.201100216