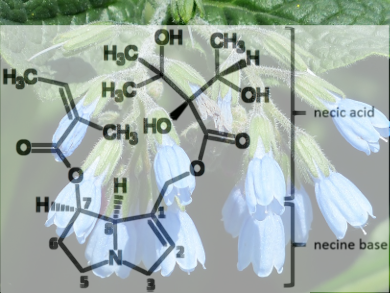
High levels of pyrrolizidine alkaloids (PA) have repeatedly been detected in herbal teas and honey. PA are a group of secondary metabolites of plants. Certain plants of the families of Boraginaceae, Asteraceae, Apocynaceae, and Fabaceae produce them as defensive compound against herbivores. More than 660 PA and PA-N oxides (PANO) occur in more than 6,000 plant species worldwide; about 3 % of the world’s flora. Domestic medicinal and culinary herbs such as comfrey (Symphytum spp; pictured.) and borage (Borago officinalis) contain these alkaloids. The alkaloid-containing plants thrive as weeds between crops. They get into the food chain, especially by mechanical harvesting. PA-containing plants taste so bitter that grazing animals usually avoid them. As an admixture of feeds such as hay, silage, or pressed dry pellets (cobs), however, the animals no longer perceive them. The eye-catching blossoms of PA plants are the target of honey bees.
Christoph Gottschalk, Manfred Gareis, and Florian Kaltner, Ludwig Maximilian University of Munich, Germany, looked at the chemistry of PA and PANO. PA and PANO are bicyclic tertiary amines or their N-oxides, also called necine bases, which are esterified with branched polyhydroxy carboxylic acids (necine acids). Only 1,2-unsaturated PA and PANO, which are hydroxylated at positions C7 and C9 and esterified with at least one necine acid, are poisonous. PA/PANO are considered to be protoxic: After consumption, enzymes in the liver convert the substances to pyrrole esters. In this metabolically activated form, protein and DNA adducts are formed. They damage the liver and other organs in both acute and chronic PA/PANO uptake.
The analysis of the substances is complex because they are diverse, exist in isomeric structures, and have a wide range of polarity. Little is known about the stability of the compounds in different matrices. Current methods for PA analysis mainly use LC-MS/MS. Two different approaches are used: The determination in sum or the individual determination. The disadvantages of the determination in sum are the time-consuming sample preparation and the difficulty to determine the toxicity of single PA; the disadvantages of the individual determination are the limited availability of commercially available PA/PANO reference standards, their high costs, and the time required for the evaluation of all analytes for each sample.
To assess the risk of PA contamination in the food chain, the data needs to be improved. In addition, scientific work is required to elucidate the structure-activity relationships of different PA groups and to derive their toxicological relevance. Legislative maximum levels of PA/PANO in food and feed are only to be expected if sufficient data on toxicology and exposure are available and recommendations are made for a standardized analytical method (sum or single analyte methods).
- Pflanzeninhaltsstoffe als Kontaminanten in der Lebensmittelkette,
Florian Kaltner, Christoph Gottschalk, Manfred Gareis,
Nachr. Chem. 2018.
https://doi.org/10.1002/nadc.20184067856