Frog Pheromones
Amphibians are at home in water, but can they also sense volatile compounds in the air? “Indeed they can,” reports Stefan Schulz. Working with colleague Miguel Vences and Ph.D. students Dennis Poth and Katharina Wollenberg at the University of Brunswick, Germany, he has found volatile pheromones in frogs from Madagascar. In the journal Angewandte Chemie, the scientists have now introduced various natural compounds that the frogs apparently use for communication.
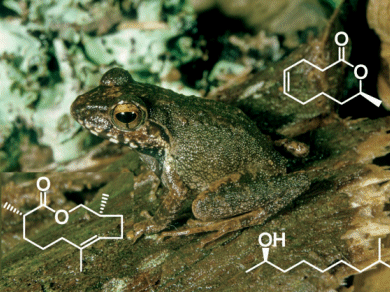
“Anuran amphibians communicate primarily by means of acoustic, optical, and tactile signals,” explains Schulz. “In addition, they also seem to communicate through peptides and proteins that easily dissolve in water or on the water’s surface. There have recently been indications that frogs may also respond to volatile signal compounds.” Schulz and his co-workers have now examined frogs from Madagascar (Mantellidae), a very species-rich family of frogs from the rainforests. The males of one subspecies, the Mantellinae, form large characteristic glands on the undersides of their rear shanks. The function of these glands was not previously known, but they could be related to pheromonal communication. Schulz and his colleagues now report a surprising discovery: “The glands contain volatile, nonpeptidic compounds that act as pheromones and are structurally related to volatile insect secretions.”
Chemical Recognition
In the glands of the frog Mantidactylus multiplicatus, the researchers found two volatile main components, and demonstrated that the frogs react to both substances. One of the components is an alcohol, the other a macrolide, a ring-shaped molecule with an intramolecular ester group. It is related to phoracantholide J, a component of the defensive secretion of the Australian beetle Phoracantha synonyma. However, the spatial arrangement of the atoms is different: the frog macrolide is the mirror image of the beetle molecule. For identification purposes, Schulz’s team developed a new synthetic route for the production of phoracantholide J that delivers enantiomerically pure products, either only the original version or the mirror image. Their method is also less complicated than earlier approaches.
The researchers found similar macrolides in the glands of related frogs. For example, in the species Gephyromantis boulengeri, they discovered a previously unknown macrolide that they named gephyromantolid A. “In fact, volatile compounds are widespread among the Mantellinae, but occur in species-specific mixtures,” says Schulz. “The volatile compounds could play a previously underrated role in species recognition over short distances in these very species-rich communities.” This could explain the extreme degree of species diversity of frogs in the tropical rainforest. With over 100 species per region in Madagascar, chemical recognition of species could help to avoid failed pairings that lead to nonviable offspring. Such macrolides could thus have a significant influence on the speciation and evolution of tropical amphibians.
- Volatile Amphibian Pheromones: Macrolides of Mantellid Frogs From Madagascar,
D. Poth, K. C. Wollenberg, M. Vences, S. Schulz,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201106592