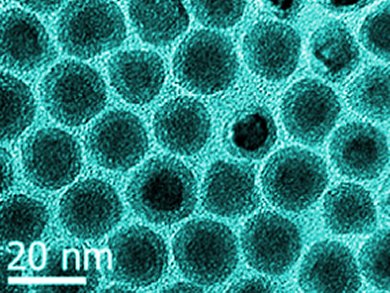
Biocompatible nanoparticles that exhibit a high magnetic moment have potential uses for a variety of biomedical applications such as targeted drug delivery, hyperthermia, and MRI contrast enhancement. Owing to the reactive nature of iron with water and oxygen, the preparation of stable iron nanoparticles is highly challenging. As iron has a much higher saturation magnetization than iron oxide, it is desirable to retain the iron core for better magnetic performance.
Richard Tilley and co-workers, Victoria University of Wellington, New Zealand, report an elegant synthesis of monodisperse biocompatible core/shell nanoparticles, which have an iron core with high saturation magnetization and a shell of iron oxide. In the presence of excess stabilizer, the synthesized particles are resistant to oxidation over time and their magnetic properties are preserved. The authors used Mössbauer spectroscopy to identify the iron oxide species present in the shell layer as principally γ-Fe2O3.
These core/shell nanoparticles (pictured) were used as cellular magnetic resonance imaging contrast agents and exhibited enhancement compared to iron oxide nanoparticles with similar cytotoxicity. This outcome means that these particles are promising candidates for biomedical applications.
Image: © Wiley-VCH
- Synthesis and Stability of Highly Crystalline and Stable Iron/Iron Oxide Core/Shell Nanoparticles for Biomedical Applications,
S. Cheong, P. Ferguson, I. F. Hermans, G. N. L. Jameson, S. Prabakar, D. A. J. Herman, R. D. Tilley,
ChemPlusChem 2012.
DOI: 10.1002/cplu.201100074
This article is available for free as part of the ChemPlusChem free trial.