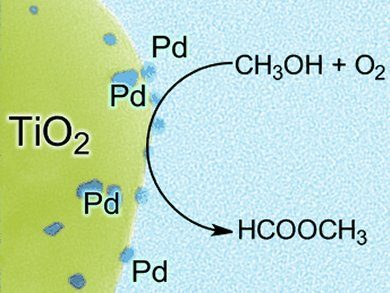
The gas phase partial oxidation of methanol to methyl formate usually requires high temperatures of > 250 °C to control the product selectivity. By using supported palladium nanoparticles, Robert Wojcieszak and co-workers, Université Catholique de Louvain, Belgium, have defied this norm and developed the low-temperature, highly selective production of methyl formate from methanol. Methyl formate was produced with selectivities of up to 100 % at temperatures between 50 and 100 °C.
Pd/TiO2 catalysts were prepared by using a water-in-oil microemulsion, which involves chemical reduction then precipitation from solution and allows for fine control of particle size. Nanoparticle size and distribution optimization showed decreasing particle size correlated strongly with increasing methanol conversion. Decreasing particle size can induce new physical and chemical properties. A small-particle catalyst has a higher quantity of exposed active sites and thus a higher activity. After a certain threshold, quantum effects come into play, which can give surprising properties.
This research suggests that the palladium nanoparticles (1–8 nm) induce C–O–C coupling at low temperature.
Image: © Wiley-VCH
- Low Temperature-High Selectivity Process over Supported Pd Nanoparticles in Partial Oxidation of Methanol,
R. Wojcieszak, E. M. Gaigneaux, P. Ruiz,
ChemCatChem 2012, 4, 72–75.
DOI: 10.1002/cctc.201100215