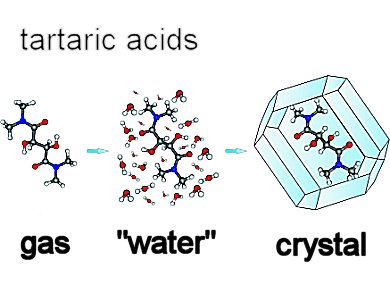
Tartaric acid is one of the most important organic compounds existing in nature and rightfully called a “lab animal” for its chemical history. Whereas the structure of natural (R,R)-tartaric acid has been the subject of several studies in the past, its conformation and the conformations of its derivatives remained a less known property. Even less is known about the structure of the meso stereoisomer, (R,S)-tartaric acid.
Jacek Gawronski and colleagues, A. Mickiewicz University, Poznan, Poland, show that the structure of polar molecules such as tartaric acids and their derivatives is primarily controlled by the possibility to form multiple hydrogen bonds (HBs) under given conditions.
Using DFT calculations, spectroscopic measurements, and X-ray diffraction, they determine the controlling factors for conformational changes of the tartaric acids and their derivatives in vacuo, in solution, and in the crystalline state. All structural variations can be logically accounted for by the possibility of formation and breaking of hydrogen bonds between the hydroxy or amide donors and oxygen acceptors. Among these are the hydrogen bonds that close five-membered rings the most stable.
These findings are useful in designing molecular and crystal structures of highly polar, polyfunctional, chiral compounds.
- From Single Molecule to Crystal: Mapping Out the Conformations of Tartaric Acids and Their Derivatives,
Agnieszka Janiak, Urszula Rychlewska, Marcin Kwit, Urszula Stepien, Krystyna Gawronska, Jacek Gawronski,
ChemPhysChem 2012.
DOI: 10.1002/cphc.201200033