A Simple, Cheap, and Unpowered Test
Complex laboratory investigations do produce reliable results, but they are not useful for point-of-care diagnostics. This is especially true in developing countries, which must rely on simple, inexpensive test methods that do not require a power source. Biosensors based on paper are an interesting alternative. American researchers from the University of Texas at Austin and the University of Illinois at Urbana-Champaign have now introduced a particularly clever concept in the journal Angewandte Chemie: print on one side of the paper, fold it up origami-style, laminate it, and the test is ready. Test evaluation requires only a voltmeter.
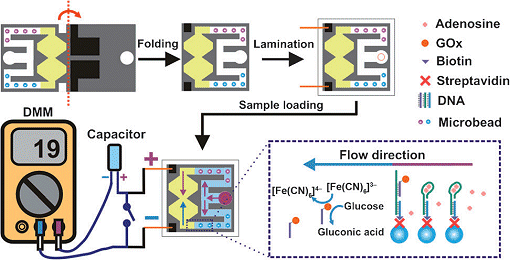
The team of researchers uses chromatography paper fabricated by wax printing. The printed areas become hydrophobic, while the unprinted paper remains hydrophilic. On one half of the paper, the researchers led by Richard M. Crooks and Hong Liu created a sample inlet and two hydrophilic channels, each leading from the inlet to a small chamber. The two chambers are connected to each other through a narrow opening. The required reagents are also “printed” onto the paper. On the second half of the paper, a screen-printing process is used to add two electrodes made of conductive carbon ink. When the paper is then folded down the middle according to the principles of origami—no tape or glue—a three-dimensional structure is formed. This causes the electrodes to come into contact with the chambers. Finally, the folded paper is laminated.
Two Channels Create Easy-to-Detect Potential Difference
When a drop of the sample is put into the inlet, the liquid moves through the two channels. One of the channels contains microspheres coated with an aptamer. An aptamer is a strand of DNA that can be constructed so as to selectively bind nearly any desired analyte molecule. For the purpose of demonstration, the researchers chose an aptamer for adenosine. If adenosine is in the sample, the aptamer binds to it. This releases an enzyme that was coupled to the aptamer. The enzyme continues to flow through the channel and reaches the chamber, which contains glucose and Prussian blue (iron hexacyanoferrate). This complex contains trivalent iron. The enzyme, glucose oxidase, oxidizes the glucose, which causes the iron in the Prussian blue to be reduced to the divalent form.
The second channel contains spheres with no aptamer. In the second chamber, therefore, no iron is reduced. Because the oxidation state of the iron in one chamber has been changed, the two chambers no longer have the same composition and an electric potential builds up. This can be measured by means of a capacitor and a measuring device like those used to test the voltage of a battery.
This principle can be used to easily and inexpensively produce rapid tests for a broad spectrum of different target molecules.
Images: © Wiley-VCH
- Aptamer-Based Origami Paper Analytical Device for Electrochemical Detection of Adenosine,
Hong Liu, Yu Xiang, Yi Lu, Richard M. Crooks,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201202929



