Aromatic carbonates, mainly diphenyl carbonate (DPC), are important materials used for the industrial production of polycarbonates (PCs) by a melt polymerization process. Traditionally, PCs have been produced through the phosgene process, in which interfacial polymerization occurs between bisphenol-A and phosgene. As this process uses large amounts of methylene chloride as the solvent, as well as highly toxic phosgene as a reactant, the substitution of this process with a more environmentally friendly process has driven research.
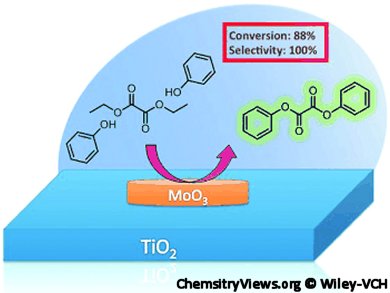
Shubhangi Umbarkar, National Chemical Laboratory, Pune, India, and, Franck Dumeignil, CNRS Unitè de Catalyse et Chimie du Solide, Villeneuve d’Ascq, France, and colleagues report the use of a new sol–gel technique for the synthesis of MoxTi catalysts; titania loaded with x % MoO3. They used titanium peroxide and molybdenum peroxide as precursors. A series of catalysts with various Mo loadings has been tested for the transesterification of diethyl oxalate (DEO) and phenol to diphenyl oxalate (DPO). Remarkably, 100 % selectivity for DPO was observed, irrespective of the Mo loading, with a maximum conversion of 88 % for 1 wt % MoO3 catalyst. .jpg)
The stability of the system was exemplified by the recycling and reuse of one formulation three times without a drop in conversion.
- Transesterification of Diethyl Oxalate with Phenol over Sol–Gel MoO3/TiO2 Catalysts,
Trupti Kotbagi, Duy Luan Nguyen, Christine Lancelot, Carole Lamonier, Kaew-Arpha Thavornprasert, Zhu Wenli, Mickael Capron, Louise Jalowiecki-Duhamel, Shubhangi Umbarkar, Mohan Dongare, Franck Dumeignil,
ChemSusChem 2012.
DOI: 10.1002/cssc.201100802