In organic chemistry, hyperconjugation is responsible for a variety of phenomena, including the anomeric effect, gauche effect, β-silicon effect, stability of carbocations and free radicals.
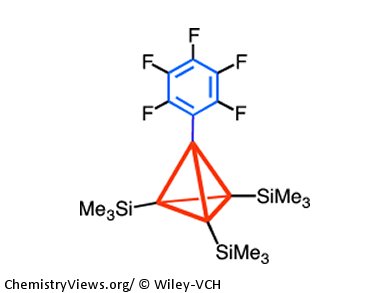
To visualize the effects of hyperconjugation, Akira Sekiguchi, University of Tsukuba, Japan, and colleagues synthesized tetrakis(trimethylsilyl)tetrahedrane 1 and its pentafluorophenyl derivative 2 and recorded their ultraviolet photoelectron spectra (UV-PES).
tetrahedrane.gif)
The observation of an energy gap of 2.0 eV between the first and fourth ionization bands of 2 provides clear-cut evidence for the σtetrahedrane–πarene orbital interaction (neutral hyperconjugation). This nicely supports the preliminarily proposed neutral hyperconjugation in 2, which was based on structural and spectroscopic data.
- UV-Photoelectron Spectroscopy of a Tetrakis(trimethylsilyl)-tetrahedrane and Its Pentafluorophenyl Derivative
Anna Chrostowska, Alain Dargelos, Patrick Baylère, Alain Graciaa, Yusuke Inagaki, Masaaki Nakamoto, Vladimir Ya. Lee, Akira Sekiguchi,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201300044