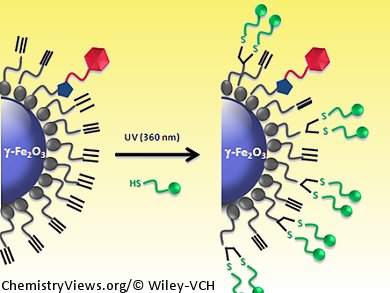
A metal-free click chemistry approach to functionalize nanoparticle surfaces has been developed by Erwann Guénin and colleagues, Université Paris and Université de Rouen, France. They have used the water-compatible radical thiol-yne reaction to add successive functional groups to the surface of a superparamagnetic γ-Fe2O3 nanoparticle.
The reaction does not require any metal catalysis, is quite fast, and is completely irreversible, unlike the related thiol-ene process. The thiol-yne reaction is compatible with the conjugation of biomolecules and it allows dendrimeric functionalization of the surfaces; under UV irradiation and in the presence of a radical initiator, two thiol moieties were added successively to one alkyne group. Because the reaction allows two consecutive additions of thiol groups on one moiety, the grafting is very efficient and a high surface density can be reached quite easily, making it highly suitable for surface chemistry
The use of the thiol-yne reaction for functionalization of nanoparticles is more efficient than using copper(I)-catalyzed azide-alkyne cycloaddition (CuACC) chemistry and higher levels of grafting are obtained. Nevertheless, the nanoparticle is also compatible with the CuAAC procedure, meaning sequential click reactions can be conducted on the nanoparticle surface and various types of molecules can be added to the surface. For example, by introducing HS-PEG-COOH (poly(ethylene glycol) 2-mercaptoethyl ether acetic acid) onto the nanoparticle surface, the possibility of a further functionalization step by carbodiimide coupling is introduced and the surface can be conveniently tailored to create a multimodal nanoplateform for fluorescence, MRI, or targeting applications.
- Nanoparticles Under the Light: Click Functionalization by Photochemical Thiol-yne Reaction, Towards Double Click Functionalization,
Paul Demay-Drouhard, Emilie Nehlig, Julie Hardouin, Laurence Motte, Erwann Guénin,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201300903