The aromatic character of “inorganic” benzene, borazine B3N3H6, is still under debate. As bond-length equalization is one criterion of aromaticity, bond-length alternation (BLA) in annulated benzenoid molecules has been investigated extensively and accurate computations of molecular structures, including those of larger molecules, for comparison with crystal structures are becoming increasingly important.
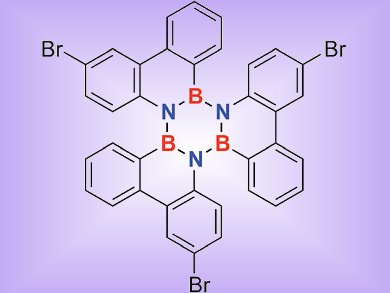
Holger Bettinger and colleagues, University of Tübingen, Germany, investigated sterically overcrowded borazines by computational and experimental means. The experimentally obtained crystal structures of 1,2:3,4:5,6-tris(biphenylylene)borazine 1 and its tribromo derivative 2 were compared with those accessed by modern density functional theory (DFT) computations.
The reported crystal structure of 1 shows a significantly larger BLA of the inner borazine core than expected based on computations. To determine whether this BLA is inherent of this borazine motif, the tribromo derivative 2 was synthesized by electrophilic aromatic bromination, a reaction that was not described previously for aryl borazines.
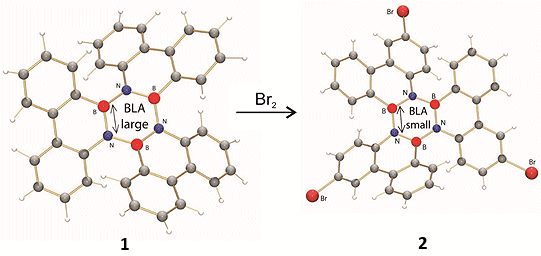
As the BLA obtained from DFT computations and the crystal structure of derivative 2 are both small, the crystal structure of the unsubstituted borazine 1 was reinvestigated. It was revealed that statistical occupation of boron and nitrogen sites – ‘positional disorder’ – within the borazine ring precludes discussions about bond lengths. Thus elucidating that bond-length alternation is significantly smaller than was reported previously.
- Is There B–N Bond-Length Alternation in 1,2:3,4:5,6-Tris(biphenylylene)borazines?
Matthias Müller, Cäcilia Maichle-Mössmer, Peter Sirsch, Holger F. Bettinger,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201300110