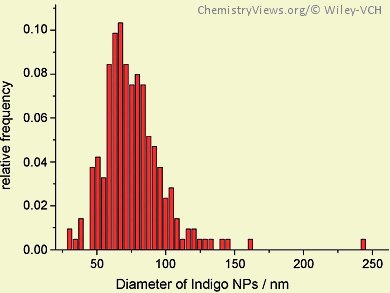
The size of nanoparticles is critical for their applications and it is thus crucial to be able to accurately measure the size distribution of nanoparticles. Techniques such as transmission electron microscopy (TEM) or scanning electron microscopy (SEM) can be used to determine the size of metal nanoparticles. However, it is difficult to measure the size of organic nanoparticles because their sizes can change after drying, their aggregation state may change, or expensive instruments and long processing times are required.
Richard G. Compton and his co-workers, University of Oxford, UK, have come up with an electrochemical method for the sizing of organic nanoparticles. Their technique is based on the Faradaic charge transfer that occurs when the nanoparticle hits an electrode. They prepared a series of indigo nanoparticles with various sizes and determined their reduction potential. Chronoamperometric profiles showed reductive spikes that result from the reduction of nanoparticles in random collision events. Analysis of the impact spikes resulted in a size distribution of the indigo nanoparticles that was derived from the charge passed during reduction, the nanoparticle density, and the relative atomic mass. Comparison of the size distribution from the electrochemical sizing method with that derived from dynamic light scattering showed an excellent agreement.
This direct electrochemical sizing technique can be used for the simultaneous sizing of individual organic nanoparticles and can thus be used where other sizing methods are unfeasible, such as in drug delivery.
- Electrochemical Sizing of Organic Nanoparticles,
Wei Cheng, Xiao-Fei Zhou, Richard G. Compton,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201307653