Skin Pigments with Technological Potential
A black and insoluble biopolymer called eumelanin and other types of melanin together determine skin and hair color, particularly for dark phenotypes. Eumelanin is also a soft, biocompatible nanomaterial with technological potential. However, previous studies of this substance have primarily been carried out with synthetic samples. In the journal Angewandte Chemie, Italian researchers have now revealed why natural eumelanin is significantly superior to the synthetic version as a radical scavenger, antioxidant, and photoprotectant.
Thanks to its unusual optoelectronic, dielectric, metal-binding, and radical-scavenging properties, eumelanin could be useful for a variety of technical applications, including organic electronic components or antioxidants for plastics. However, it was recently discovered that the properties of synthetic eumelanin are significantly different from those of the natural product.
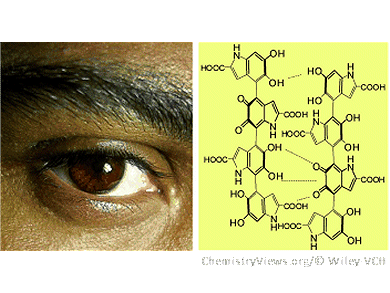
Within pigment cells, eumelanin is produced enzymatically from tyrosine or DOPA. An important intermediate step in this process is the isomerization of dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA). In contrast to the natural process, the laboratory synthesis does not use enzymes. In this case, a carboxylic acid group spontaneously splits off to give 5,6-dihydroxyindole (DHI). While natural eumelanins contain over 50 % DHICA-derived components, synthetic versions primarily consist of building blocks derived from DHI.
Natural Eumelanin versus Synthetic Eumelanin
A team at the University of Naples and the National Research Council of Italy, Pozzuoli, Italy, has now produced melanins based on DHI and DHICA, and compared them. The results demonstrated that the molecular structures and morphologies, as well as the optoelectronic and paramagnetic properties of the two variants are very different. Whereas the DHI-based polymers exist as small, spherical aggregates, the DHICA-based versions form micrometer-long rods from elongated aggregates. The DHICA polymer is a considerably stronger proton donor and radical scavenger than the DHI polymer.
The researchers, headed by Marco d’Ischia, determined that the cause of this difference is the different way in which the building blocks of the two types of melanin are attached to each other. In the DHI biopolymer, the electrons from double bonds can move freely throughout the entire molecule (conjugated double bonds). This has a stabilizing effect and results in flat molecules that aggregate into compact stacks. In the DHICA-based melanins, this conjugation between the building blocks is hindered, which has a destabilizing effect and leads to monomer-like behavior and less aggregation. Paradoxically it is precisely this destabilization that gives the DHICA melanins their unusually efficient antioxidant, redox, and photoprotectant properties– making them excellent protective skin pigments.
The researchers are optimistic that the outstanding radical-scavenging powers of the DHICA-based melanins will also prove to be superior to previously tested DHI polymers in technical applications.
- Atypical Structural and π-Electron Features of a Melanin Polymer that Lead to Superior Free-Radical-Scavenging Properties,
Lucia Panzella, Gennaro Gentile, Gerardino D’Errico, Nicola F. Della Vecchia, Maria E. Errico, Alessandra Napolitano, Cosimo Carfagna, Marco d’Ischia,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201305747