In order to achieve industrial-scale production capability, the synthesis of graphene by means of a chemical approach is crucial. Pristine graphene is, theoretically, a single layer of a conjugated sp2 carbon network arranged in a honeycomb structure with unique mechanical, optical, thermal, and electrical properties. However, it is very challenging to produce pristine graphene in large quantities.
Chemically produced graphene is more commonly obtained by the top-down approach of graphene synthesis. This route involves the oxidation of graphite to graphite oxide followed by exfoliation to give graphene oxide and its subsequent reduction to graphene. New approaches have been sought in an attempt to improve the reduction process—as well as the electrical properties—of graphene by regenerating the sp2-conjugated system or selectively removing key oxygen-containing groups that could potentially decrease the performance of the graphene.
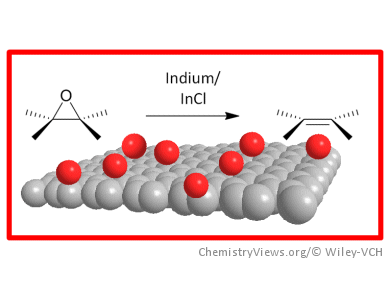
Chun Kiang Chua and Martin Pumera, Nanyang Technological University, Singapore, have shown that indium and indium(I) chloride can be successfully used for the selective removal of epoxide groups to regenerate the sp2-conjugated system of graphene oxide. The application of indium reagents in this work takes a different unique approach to improve the quality of reduced graphene by regenerating sp2-hybridized carbon on graphene oxide that is heavily populated with 1,2-epoxide groups. This method can be applied independently or in conjunction with other reducing agents to further improve the quality of chemically reduced graphene.
- Regeneration of a Conjugated sp2 Graphene System through Selective Defunctionalization of Epoxides by Using a Proven Synthetic Chemistry Mechanism,
C. K. Chua, M. Pumera,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304131