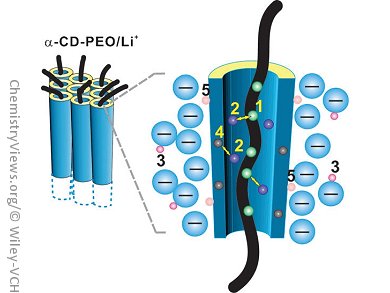
Researchers in China have created a new electrolyte that consists of cyclodextrin nanochannels. Ya-Feng Yao, Qun Chen, and their co-workers, East China Normal University, Shanghai, set out to improve the conductivity of solid polymer electrolytes based on a polyethylene oxide (PEO)/Li+ complex salt. They realized that the Li+ ion transportation would be more efficient if the size of the channel were increased and the cations and anions were kept well separated, and used α-cyclodextrin (α-CD) to form nanochannels.
This approach means that the motion of Li+ ions can take place but the cations and anions are separated. Electrochemical impedance spectroscopy was used to show that the conductivity of the nanochannel material is 30 times higher than that of the corresponding PEO/Li+ complex. The researchers also used NMR spectroscopy to investigate the dynamics of the Li+ ions in the channels, and found that the ionic motion is coupled to the segmental motion of the PEO chains.
This use of nanochannels to provide a pathway for Li+ ion motion and keep the cations and anions separated has opened a new category of crystalline polymer electrolytes.
- Transferring Lithium Ions in Nanochannels: A PEO/Li+ Solid Polymer Electrolyte Design,
Ling-Yun Yang, Da-Xiu Wei, Min Xu, Ye-Feng Yao, Qun Chen,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201307423