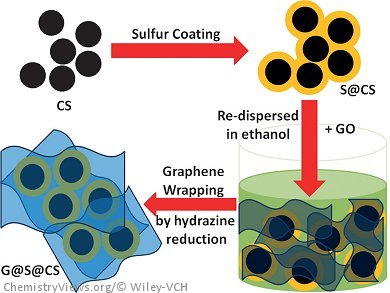
Research on lithium–sulfur batteries is increasing as sulfur is capable of delivering an ultrahigh theoretical capacity of 1685 mAh g–1 upon full reduction of sulfur to lithium sulfide (S8+16 Li ↔ 8 Li2S), surpassing all the conventional cathode materials in lithium-ion batteries. However, challenges remain in the insulating nature of sulfur and its dramatic loss in the form of polysulfide during electrochemical charge and discharge processes.
Lianzhou Wang, University of Queensland, Brisbane, Australia, and colleagues report a new type of sandwiched carbon network in which sulfur is loaded onto carbon nanospheres, which are then wrapped in graphene sheets. The graphene sheets restrain the sulfur and protect the formed polysulfide within the well-built carbon network, thereby preventing sulfur loss during electrochemical activities. The sheets also provide intimate contact with sulfur as a highly conducting medium and can buffer the side effects associated with volume expansion of the active sulfur within the battery cell during charge/discharge processes to achieve a durable service life.
When the prepared composite was applied as the lithium–sulfur cathode, the highest specific discharge capacity of 1394 mAh g–1 was obtained at a current rate of 0.1 C, and the cycling stability was well maintained for up to 100 cycles. This composite was also able to deliver specific capacities of 746 and 604 mAh g–1 at 1 C and 2 C, respectively.
- Dual Protection of Sulfur by Carbon Nanospheres and Graphene Sheets for Lithium–Sulfur Batteries,
Bei Wang, Yanfen Wen, Delai Ye, Hua Yu, Bing Sun, Guoxiu Wang, Denisa Hulicova-Jurcakova, Lianzhou Wang,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201400385